Abstract
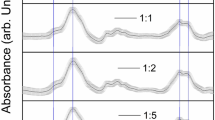
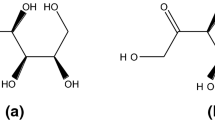
This study investigated the enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and the formation of melanoidins as a result of the Maillard reaction. The study measured reducing sugars and L- and D- amino acids using HPLC as an index for the amount of enolization of the sugars and isomerization of the amino acids. Additionally, the absorption of melanoidins was measured at different wavelengths (420, 450, 470, 490 nm); the UV–Vis spectra and the extinction coefficient were determined for the formation of melanoidins. Melanoidins were, rather arbitrarily, defined by a high-molecular-weight (HMW) if it was above a lower limit of 12.4 kDa, which was the nominal cut-off value in the dialysis system used. A remarkable enolization reaction of the sugars was observed in the course of the Maillard reaction. Especially, in the Fru/D-Asn model system, the degree of sugar enolization was more than in the other model systems. All of the FDAA (1-fluoro-2, 4-dinitrophenyl-5-L-alanine amide) amino acids were separated by TLC. The racemization of the amino acids was higher in the fructose-amino acids systems. Isomer formation was the highest in the Fru/D-Asn system. The L- and D- isomers showed different absorptions in the UV–Vis spectra, although these had similar shapes. The absorption of the melanoidins formed from glucose was higher than that formed from fructose. In particular, the sugar–asparagine system showed different characteristics according to the L- and D-isomers. The differences in the extinction coefficients of the melanoidins was significant (P < 0.05), except for the sugar–lysine system.





Similar content being viewed by others
References
Ajandouz EH, Tchiakpe LS, DalleOre F, Benajiba A, Puigserver A (2001) Effect of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J Food Sci 66:926–931
Anet EFLJ (1964) 3-deoxyglycosuloses (3-deoxyglycosones) and the degradation of carbohydrates. Adv Carbohydr Chem 19:181–218
Bada J L (1972) Kinetics of racemization of amino acids as a function of pH. J Am Chem Soc 94:1371–1373
Berg H E, Van Boekel MAJS (1994) Degradation of lactose during heating of milk. I. Reaction pathways. Neth Milk Dairy J 48:157–175
Bhushan R, Brückner H (2004) Marfey’s reagent for chiral amino acid analysis: a review. Amino Acids 27:231–247
Bhushan R, Martens J (1997) Direct resolution of enantiomers by impregnated TLC. Biomed Chromatogr 11:280–285
Bhushan R, Martens J (2003) Amino acids and their derivatives. In: Sherma J, Fried B (eds) Hand book of thin layer chromatography. Marcel Dekker Inc, New York, pp 373–415
Brands C, Wedzicha B, Van Boekel MAJS (2002) Quantification of melanoidin concentration in sugar–casein systems. J Agric Food Chem 50:1178–1183
Brückner H, Justus J, Kirschbaum J (2001) Saccharide induced racemization of amino acids in the course of the Maillard reaction. Amino Acids 21:429–433
Brückner H, Schieber A (2000) Determination of free D-amino acids in mammalian by chiral gas chromatography–mass spectrometry. J High Resol Chromatogr 23:576–582
Casal S, Mendes E, Oliveira MBPP, Ferreira MA (2005) Roast effects on coffee amino acid enantiomers. Food Chem 89:333–340
Delgado-Andrade C, Morales FJ (2005) Unravelling the contribution of melanoidins to the antioxidant activity of coffee brews. J Agric Food Chem 53:1403–1407
Erbe T, Brückner H (2000a) Chromatographic determination of amino acids enantiomers in beers and raw materials used for their manufacture. J Chromatogr A 881:81–91
Erbe T, Brückner H (2000b) Studies on the optical isomerization of dietary amino acids in vinegar and aqueous acetic acid. Eur Food Res Technol 21:6–12
Feather MS (1985) Some aspects of the chemistry of non-enzymatic browning (Maillard reaction). In: Chemical changes in food during processing. AVI Publ. Co, New York, pp 289–303
Friedman M (1996) Food browning and its prevention: an overview. J Agric Food Chem 44:631–653
Friedman M (1999) Chemistry, nutrition, and microbiology of D-amino acids. J Agric Food Chem 47:3457–3479
Fujimaki M, Homma S, Arakawa N, Inagaki C (1979) Growth response of rats fed with a diet containing nondialyzable melanoidin. Agric Biol Chem 43:497–503
Guimaraes C, Bento LSM, Mota M (1996) A study of sugar colourants through ion exchange and salt regeneration. Int Sugar J 98:584–587
Hashiba H (1982) The browning reaction of Amadori compounds derived from various sugars. Agric Biol Chem 46:547–548
Hofmann T (1998) Studies on the relationship between molecular weight and the color potency of fractions obtained by thermal treatment of glucose/amino acid and glucose/protein solutions by using ultracentifugation and color dilution techniques. J Agric Food Chem 46:3891–3895
Joseph MH, Marsden CA (1986) Amino acids and small peptides. In: Lim CK (ed) HPLC of small molecules: a practical approach. IRL, Oxford, pp 13–28
Kutz N, Pätzold R, Brückner H (2004) Bestimmung von Aminosäureenantiomeren in Kakao und Kakaoprodukten. Lebensmittelchemie 58:106–107
Ledl F, Schleicher E (1990) New aspects of the Maillard reaction in foods and in the human body. Angew Chem Int Ed 29:565–594
Lee YS, Homma S, Aida K (1987) Characterization of melanoidin in soy sauce and fish sauce by electrofocusing and high performance gel permeation chromatography. Nippon Shokuhin Kogyo Gakkaishi 34:313–319
Leong LP (1999) Modelling the Maillard reaction involving more than one amino acid. PhD thesis, Procter Department of Food Science, University of Leeds, UK
Leong LP, Wedzicha BL (2000) A critical appraisal of the kinetic model for the Maillard browning of glucose and glycine. Food Chem 68:21–28
Maillard LC (1913) Formation de matieres humiques par action de polypeptides sur sucres. C R Acad Sci 156:148–149
Marfey P (1984) Determination of D-amino acids. II. Use of a bifunctional reagent, 1, 5-difluoro-2, 4-dinitrobenzene. Carlsberg Res Commun 49:591–596
Morales FJ, Fernandez-Fraguas C, Jiménez-Pérez S (2005) Iron binding ability of melanoidins from food and model systems. Food Chem 90:821–827
Morales FJ, Jiménez-Pérez S (2004) Peroxyl radical scavenging activity of melanoidins in aqueous systems. Eur Food Res Technol 218:515–520
Morales FJ, Romero C, Jiménez-Pérez S (1996) Evaluation of heat-induced changes in Spanish commercial milk: hydroxymethylfurfural and available lysine content. Int J Food Sci 31:411–418
Nagata Y, Lidat T, Sakai M (2001) Enantiomeric resolution of amino acids by thin-layer chromatography. J Mol Catal B Enzym 12:105–108
Nagata Y, Masui R, Akino T (1992) The presence of free D-serine, D-alanine and D-proline in human plasma. Experientia 48:986–988
Pätzold R, Brückner H (2004) Mechanistische aspekte und konsequenzen der bildung von D-aminosäuren im laufe der Maillardreaktion. Lebensmittelchemie 58:100
Pätzold R, Brückner H (2005) Mass spectrometric detection and formation of D-amino acids in processed plant saps, syrups, and fruit juice concentrates. J Agric Food Chem 53:9722–9729
Pätzold R, Brückner H (2006a) Gas chromatographic detection of D-amino acids in natural and thermally treated bee honeys and studies on the mechanism of their formation as result of the Maillard Reaktion. Eur Food Res Technol 223:347–354
Pätzold R, Brückner H (2006b) Gas chromatographic determination and mechanism of formation of D-amino acids occurring in fermented and roasted cocoa beans, cocoa powder, chocolate and other cocoa products. Amino Acids 31:63–72
Pätzold R, Nieto-Rodriguez A, Brückner H (2003) Chiral gas chromatographic analysis of amino acids in fortified wines. Chromatographia 57(Suppl):S207–S212
Pirkle WH, Pochapsky TC (1989) Considerations of chiral recognition relevant to the liquid chromatographic separation of enantiomers. Chem Rev 89:347–362
Rafik M, Mas A, Elharfi A, Schue F (1997) Decoloration de solutions sucrees par ultrafiltration sur une membrane a base de poly(organocyclophosphazene). Eur Polymer J 33:679–690
Rizzi GP (1997) Chemical structure of coloured Maillard reactions products. Food Rev Int 13:1–28
Roth M (1971) Fluorescence reaction for amino acids. Anal Chem 43:880–882
Rufián-Henares JA, Morales FJ (2005) A screening procedure to assess the antimicrobial activity of melanoidins. Oral presentation at COST Action 919, Melanoidins in food and health, Hamburg, Germany, July 2004
Sara IFSM (1997) Kinetic modelling of glucose and glycine. Graduation thesis, Technical University of Lisbon, Portugal and Wageningen University, The Netherlands
Sara IFSM, Van Boekel MAJS (2003) Melanoidins extinction coefficient in the glucose/glycine Maillard reaction. Food Chem 83:135–142
Speck JCJ (1958) The Lobry de Bruyn-Alberda van Ekenstein transformation. Adv Carbohydr Chem 13:63–103
Szókán G, Mezo G, Hudecz F (1988) Application of Marfey’s Reagent in racemization studies of amino acids and peptides. J Chromatogr 444:115–122
Tressl R, Nittka C, Kersten E (1995) Formation of isoleucine-specific Maillard products from [1–13C]-D-glucose and [1–13C]-D-fructose. J Agric Food Chem 43:1163–1169
Van Boekel MAJS (1996) Kinetic modelling of sugar reactions in heated milk-like systems. Neth Milk Dairy J 50:245–266
Vigo MS, Malec LS, Gomez RG, Llosa RA (1992) Spectrophotometric assay using o-phthaldiadehyde for determination of reactive lysine in dairy products. Food Chem 44:363–365
Wedzicha BL, Kaputo MT (1992) Melanoidins from glucose and glycine: composition, characteristics and reactivity towards sulphite ion. Food Chem 43:359–367
Wijewickreme AN, Kitts DD, Durance TD (1997) Reaction conditions influence the elementary composition and metal chelating affinity of nondialyzable model Maillard reaction products. J Agric Food Chem 45:4577–4583
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-511-C00133)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, JS., Lee, YS. Enolization and racemization reactions of glucose and fructose on heating with amino-acid enantiomers and the formation of melanoidins as a result of the Maillard reaction. Amino Acids 36, 465–474 (2009). https://doi.org/10.1007/s00726-008-0104-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0104-z