Abstract
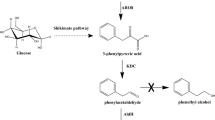
Catalyzed by phenylalanine ammonia-lyase from Rhodotorula glutinis, 2% trans-cinnamic acid and 0.5 mol/l (15NH4)2SO4 was bioconverted to 15NL-phenylalanine. The yield and the purity of 15NL-phenylalanine reached 71 and 99.3%, respectively. The results showed that 96% of 15N was labeled on the l-phenylalanine and 88% of (15NH4)2SO4 was recovered. The present paper provides a new and economic way for biosynthesis of 15NL-phenylalanine.

Similar content being viewed by others
References
Hachey DL (1994) Stable isotopes for measurement of nutrient dynamics during pregnancy and lactation. Adv Exp Med Biol 352:265–278
Hadener A, Tamm Ch (1987) Synthesis of specifically labelled l-phenylalanines using phenylalanine ammonia lyase activity. J Label Compounds Radiopharma 24(11):1291–1306
Hodgins DS (1968) The presence of a carbonyl group at the active site of l-phenylalanine ammonia-lyase. Biochem Biophys Res Co 32(2):246–253
Kenjiro T, Tetsuo K, Michinori W, Sannamu L, Yasushi K, Nobuo I (1984) Facile synthesis of (2R,3R)-phenylalanine-2,3-d 2 and NMR study on Deuterated Gramicidin S. Bull Chem Soc Jpn 57(8):2193–2197
LeMaster DM, Cronan JE (1982) Biosynthetic production of 13C labeled amino acids with site specific enrichment. J Biol Chem 257:1224–1229
Marc Y, Itzhak N (1995) Stable isotopes as metabolic probes for nutritional studies in neonates. Clin Perinatol 22(1):97–109
Onishi N, Yokozeki K, Hirose Y, Kubota K (1987) Enzymatic production of l-phenylalanine from trans-cinnamic acid by Endomyces lindneri. Agric Biol Chem 51(1):291–292
Rulin F (1986) Organic syntheses with stable isotopes. Chemical industry press, Beijing, 260 pp
Tachibana Y, Ando M (1983) A kinetic study of a Zn[2+]-catalyzed transamination reaction between pyridoxamine analogs with a pyridinophane structure and α-keto acids. Bull Chem Soc Jpn 56:3652–3656
Yamada S, Nabe K, Izuo N, Nakamichi K, Chibata I (1981) Production of the l-phe from trans-cinnamic acid with R. glutinis containing l-phe ammonia-lyase activity. Appl Environ Microbiol 42(5):773–778
Acknowledgments
This research has been supported financially by Natural Science Foundation of China (NSFC) (No.20576010). In addition, the authors are grateful for Shanghai Research Institute of Chemical Industry for providing (15NH4)2SO4 and analyses of products.
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed to the work equally.
Rights and permissions
About this article
Cite this article
Wang, W., Yue, H., Yuan, Q. et al. Biosynthesis of 15NL-phenylalanine by phenylalanine ammonia-lyase from Rhodotorula glutinis . Amino Acids 36, 231–233 (2009). https://doi.org/10.1007/s00726-008-0056-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0056-3