Summary.
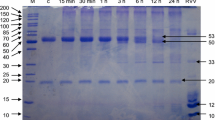
In normal and pathological tissues, elastin-derived peptides proceed of elastin degradation by polymorphonuclear leukocyte proteases: elastase, cathepsin G and proteinase 3. They were demonstrated to have a chemotactic activity, to promote cell proliferation and protease release, . . .. To be biologically active, their structures, which reflect elastase specificity, must adopt a β-turn conformation which accommodate to the cell surface-located elastin binding protein. In this study, we establish that human elastin exon 24-derived peptides containing at least two repeated VGVAPG sequences are hydrolyzed by the proteinase 3 (Pr3). As shown by mass spectrometry analyses, the demonstrated cleavage sites are in agreement with previously reported Pr3 substrate specificity and its lengthy substrate binding site. The characterization of the Pr3-generated products indicate that they contain at least one GXXPG sequence known to stimulate cellular effects after binding to the elastin receptor.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lombard, C., Bouchu, D., Wallach, J. et al. Proteinase 3 hydrolysis of peptides derived from human elastin exon 24. Amino Acids 28, 403–408 (2005). https://doi.org/10.1007/s00726-005-0192-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-005-0192-y