Abstract
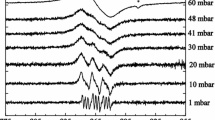
A simple model is proposed that semi-quantitatively explains the dependence of the EPR signal intensity of adsorbed NO molecules on the adsorption value. It is assumed that there are only two types of NO adsorption sites on each facet of the microcrystal, such that only NO molecules adsorbed on one of them are active in EPR. Within the framework of this model of lattice adsorption, it is assumed that there are Z other adsorption centers from the set under consideration that are available for the adsorption of neighboring NO molecules in the local environment of each EPR-active adsorption center. In this case, the formation of diamagnetic (NO)2 dimers containing an NO molecule adsorbed on a center active in the EPR decreases the integral intensity of the EPR signal. Analytical expressions are obtained for the dependence of the EPR signal intensity on the surface coverage. They were used to analyze experimental data on the adsorption of NO at the Lewis acid sites of sulfated zirconia. The proposed model consistently explains the results of EPR experiments.




Similar content being viewed by others
References
P.H. Kasai, R.J. Bishop Jr., in Zeolite Chemistry and Catalysis, ed. by J.A. Rabo (American Chemical Society, Washington DC, 1976), pp. 350–391
P.H. Kasai, R.J. Bishop, J. Am. Chem. Soc. 94, 5560 (1972)
A. Gutsze, M. Plato, H.G. Karge, F. Witzel, J. Chem. Soc. Faraday Trans. 92, 2495 (1996)
J.H. Lunsford, J. Chem. Phys. 46, 4347 (1967)
B.M. Hoffman, N.J. Nelson, J. Chem. Phys. 50, 2598 (1969)
C.L. Gardner, M.A. Weinberger, Can. J. Chem. 48, 1317 (1970)
J.H. Lunsford, J. Phys. Chem. 74, 1518 (1970)
P.H. Kasai, R.M. Gaura, J. Phys. Chem. 86, 4257 (1982)
A. Pöppl, T. Rudolf, D. Michel, J. Am. Chem. Soc. 120, 4879 (1998)
M. Niwa, T. Minami, Y. Murakami, Bull. Chem. Soc. Jpn. 49, 565 (1976)
S. Furuyama, T. Morimoto, R. Hirasawa, J. Phys. Chem. 82, 1027 (1978)
A. Adamski, G. Djéga-Mariadassou, Z. Sojka, Catal. Today 119, 120 (2007)
J.W. Jermyn, T.J. Johnson, E.F. Vansant, J.H. Lunsford, J. Phys. Chem. 77, 2964 (1973)
A.M. Volodin, K.A. Dubkov, A. Lund, Chem. Phys. Lett. 333, 41 (2001)
P. Fisicaro, E. Giamello, G. Berlier, C. Lamberti, Res. Chem. Intermed. 29, 805 (2003)
A. Volodin, D. Biglino, Y. Itagaki, M. Shiotani, A. Lund, Chem. Phys. Lett. 327, 165 (2000)
V.A. Bolshov, A.M. Volodin, G.M. Zhidomirov, A.A. Shubin, A.F. Bedilo, J. Phys. Chem. 98, 7551 (1994)
I. Langmuir, J. Am. Chem. Soc. 40, 1361 (1918)
D. Graham, J. Phys. Chem. 57, 665 (1953)
S. Brunauer, The Adsorption of Gases and Vapors. Volume 1: Physical Adsorption (Princeton University Press, Princeton, 1945)
J. Koresh, A. Soffer, J. Colloid Interface Sci. 92, 517 (1983)
P.M. Mathias, R. Kumar, J.D. Moyer, J.M. Schork, S.R. Srinivasan, S.R. Auvil, O. Talu, Ind. Eng. Chem. Res. 35, 2477 (1996)
Acknowledgements
The authors are grateful to Dr. D. K. Efremov for the sincere interest shown in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
EPR Signal Intensity of Adsorbed NO Molecules as a Function of Surface Coverage in the Framework of the Two-Site Langmuir Isotherm Model
The experimentally observed dependence of the EPR signal intensity on the surface coverage can be qualitatively, and even semi-quantitatively, explained already within the framework of the simplest two-site model of localized Langmuir monomolecular adsorption [18,19,20,21,22]. Hereinafter, we will adhere to the notation used in [19]. For this model of monomolecular adsorption, the following expression is valid for the surface coverage θ:
Here, 0 < α ≤ 1 is the relative fraction of strong adsorption centers (that is, NO molecules adsorbed on them give an easily observed EPR signal). \(\theta_{1} = n_{1} /N\) and \(\theta_{2} = n_{2} /N\) are contributions to θ due to the fact that n1 NO molecules are adsorbed in equilibrium on the strong, and n2 NO molecules are adsorbed on the weak centers, respectively. \(N = N_{1} + N_{2} = \alpha N + (1 - \alpha )N\) is the total number of adsorption sites, N1 of which are strong and N2 are weak. K1 and K2 are the Langmuir adsorption equilibrium constants for sites 1 and 2, respectively. C is the concentration of NO (equilibrium adsorption pressure).
Let’s define the ε parameter as \(\varepsilon = K_{2} /K_{1}\), then the expression for θ2 can be rewritten as:
Further, substituting Eqs. (5) in (4), we obtain a quadratic equation with respect to θ1, the solution of which, satisfying the conditions \(\theta_{1}(\theta = 0) = 0\) and \(\theta_{1}(\theta = 1) = \alpha\), has the form:
For \(\theta \ll 1\), the following expansion of Eq. (6) in powers of θ is valid:
It makes sense to give here other useful, but not used in this article, consequences of relation Eq. (6):
At this stage, let’s formalize our model for the EPR signal intensity of adsorbed NO molecules, namely: (a) NO molecules adsorbed on weak centers are not experimentally observed due to the strong broadening of the EPR signal; (b) NO molecules adsorbed on strong adsorption centers are observed in the EPR only if all centers adjacent to them (regardless of their strength) are free of NO molecules; (c) each of the strong adsorption centers has Z neighboring centers, and each of the neighbors with probability α is a strong adsorption center and, accordingly, with a probability of 1 − α—weak. In these approximations, the number of NO molecules nEPR contributing to the EPR signal becomes less than \(n_{1} = \theta_{1} N\) and is determined by the formula:
Here (1 − θ) is the probability that the given adsorption site, adjacent to the strong center under consideration, is free of the NO molecule. Indeed, according to condition (c) formulated above, the probability w that this neighboring site contains the NO molecule is \(w = \alpha \frac{{n_{1} }}{{N_{1} }} + (1 - \alpha )\frac{{n_{2} }}{{N_{2} }} = \theta_{1} + \theta_{2} = \theta\). From (9) we get the main relation of our model:
where \(n_{{{\text{ads}}}} = \theta N = n_{1} + n_{2}\) is the total number of adsorbed NO molecules, and θ1 is determined by the formula (6). Two examples of numerical calculations performed using formulas (6) and (9) are shown in Fig. 5.
Dependence of the contribution (θ1) of NO adsorption on strong adsorption centers to the total surface coverage θ at a relative fraction of strong centers α = 0.7 and a ratio of equilibrium constants for weak and strong centers ε = 0.1. Also for cases Z = 2 (a) and Z = 4 (b), the EPR signal intensities of adsorbed NO molecules normalized to the total number of surface adsorption centers \(\frac{{n_{{{\text{EPR}}}} }}{N}\) and normalized to the current total number of adsorbed NO molecules \(\frac{{n_{{{\text{EPR}}}} }}{{n_{{{\text{ads}}}} }}\) are shown
Note that using decomposition Eq. (7), two exact relations that can be useful in analyzing the experimental dependence of nEPR on θ can be obtained:
A useful consequence of formula (12) is that for \(\varepsilon \ll \alpha\), regardless of the value of α, an approximate relationship holds:
It can also be shown that under this condition (\(\varepsilon \ll \alpha\)), and additionally \(\alpha \ge \frac{1}{Z}\), the maximum of nEPR is approximately located at \(\theta_{\max } \approx \frac{1}{Z + 1}\) (see \(\frac{{n_{{{\text{EPR}}}} }}{N}\) in Fig. 5). In the case of a small relative fraction of strong centers (\(\alpha \ll 1\)), the maximum of nEPR is located at θmax of the order of α if \(\varepsilon \ll 1\) (Fig. 6a), but it shifts to the region of higher θ when the strengths of the adsorption centers approach each other (Fig. 6b).
Dependence of the contribution (θ1) of NO adsorption on strong adsorption centers to the total surface coverage θ at a relative fraction of strong centers α = 0.01 and Z = 4. The designations are the same as in Fig. 5; ε = 0.002 (a) and ε = 0.5 (b)
Rights and permissions
About this article
Cite this article
Shubin, A.A., Volodin, A.M. Integral Intensity of the EPR Signal of NO Molecules Adsorbed on Lewis Acid Sites of Oxide Systems as a Function of Surface Coverage. Appl Magn Reson 51, 993–1003 (2020). https://doi.org/10.1007/s00723-020-01250-w
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-020-01250-w