Abstract
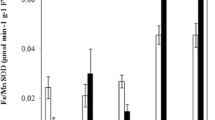
There is a very effective cross-talk between signals triggered by reactive oxygen species and hormonal responses in plants, activating proteins/enzymes likely to be involved in stress tolerance. Abscisic acid (ABA) is known as a stress hormone that takes part in the integration of signals. This work aimed to characterize the biochemical response and ultrastructural changes induced by cadmium (Cd) in the Micro-Tom (MT) sitiens ABA-deficient mutant (sit) and its wild-type (MT) counterpart. MT and sit plants were grown over a 96-h period in the presence of Cd (0, 10, and 100 μM CdCl2). The overall results indicated increases in lipid peroxidation, hydrogen peroxide content and in the activities of the key antioxidant enzymes such as catalase, glutathione reductase, and ascorbate peroxidase in both genotypes. On the other hand, no alteration was observed in chlorophyll content, while the activity of another antioxidant enzyme, superoxide dismutase, remained constant or even decreased in the presence of Cd. Roots and shoots of the sit mutant and MT were analyzed by light and transmission electron microscopy in order to characterize the structural changes caused by the exposure to this metal. Cd caused a decrease in intercellular spaces in shoots and a decrease in cell size in roots of both genotypes. In leaves, Cd affected organelle shape and internal organization of the thylakoid membranes, whereas noticeable increase in the number of mitochondria and vacuoles in MT and sit roots were observed. These results add new information that should help unravel the relative importance of ABA in regulating the cell responses to stressful conditions induced by Cd apart from providing the first characterization of this mutant to oxidative stress.









Similar content being viewed by others
References
Alcântara BK, Machemer-Noonan K, Silva Júnior FG, Azevedo RA (2015) Dry priming of maize seeds reduces aluminum stress. Plos One 10(12):e0145742. doi:10.1371/journal.pone.0145742
Ali B, Huang CR, Qi ZY, Ali S, Daud MK, Geng XX, Liu HB, Zhou WJ (2013a) 5-Aminolevulinic acid ameliorates cadmium-induced morphological, biochemical, and ultrastructural changes in seedlings of oilseed rape. Environ Sci Pollut Res 20:7256–7267. doi:10.1007/s11356-013-1735-5
Ali B, Tao Q, Zhou Y, Gill RA, Ali S, Rafiq MT, Xu L, Zhou W (2013b) 5-Aminolevolinic acid mitigates the cadmium-induced changes in Brassica napus as revealed by the biochemical and ultra-structural evaluation of roots. Ecotoxicol Environ Saf 92:271–280. doi:10.1016/j.ecoenv.2013.02.006
Al-Khateeb W, Al-Qwasemeh H (2014) Cadmium, copper and zinc toxicity effects on growth, proline content and genetic stability of Solanum nigrum L. a crop wild relative for tomato; comparative study. Physiol Mol Biol Plants 20:31–39. doi:10.1007/s12298-013-0211-5
An Y, Zhou P, Liang J (2014) Effects of exogenous application of abscisic acid on membrane stability, osmotic adjustment, photosynthesis and hormonal status of two Lucerne (Medicago sativa L.) genotypes under high temperature stress and drought stress. Crop Pasture Sci 65:274–286. doi:10.1071/cp13162
Arruda SCC, Barbosa HS, Azevedo RA, Arruda MAZ (2013) Comparative studies focusing on transgenic through cp4EPSPS gene and non-transgenic soybean plants: an analysis of protein species and enzymes. J Proteom 93:107–116. doi:10.1016/j.jprot.2013.05.039
Arruda SCC, Silva ALD, Galazzi RM, Azevedo RA, Arruda MAZ (2015) Nanoparticles applied to plant science: a review. Talanta 131:693–705. doi:10.1016/j.talanta.2014.08.050
Asgher M, Khan MIR, Anjum NA, Khan NA (2015) Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma 252:399–413. doi:10.1007/s00709-014-0710-4
Azevedo RA, Carvalho RF, Cia MC, Gratão PL (2011) Sugarcane under pressure: an overview of biochemical and physiological studies of abiotic stress. Trop Plant Biol 4:42–51. doi:10.1007/s12042-011-9067-4
Barbosa HS, Arruda SCC, Azevedo RA, Arruda MAZ (2012) New insights on proteomics of transgenic soybean seeds: evaluation of differential expressions of enzymes and proteins. Anal Bioanal Chem 402:299–314. doi:10.1007/s00216-011-5409-1
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cai BD, Yin J, Hao YH, Li YN, Yuan BF, Feng YQ (2015) Profiling of phytohormones in rice under elevated cadmium concentration levels by magnetic solid-phase extraction coupled with liquid chromatography tandem mass spectrometry. J Chromatogr A 1406:78–86. doi:10.1016/j.chroma.2015.06.046
Carvalho RF, Campos ML, Pino LE, Crestana SL, Zsögön A, Lima JE, Benedito VA, Peres LEP (2011) Convergence of developmental mutants into a single tomato model system: ‘Micro-Tom’ as an effective toolkit for plant development research. Plant Methods 7:18. doi:10.1186/1746-4811-7-18
Carvalho RF, Monteiro CC, Caetano AC, Dourado MN, Gratão PL, Haddad CKB, Peres LEP, Azevedo RA (2013) Leaf senescence in tomato mutants as affected by irradiance and phytohormones. Biol Plant 57:749–757. doi:10.1007/s10535-013-0333-1
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679. doi:10.1146/annurev-arplant-042809-112122
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K (2010) Cadmium stress: an oxidative challenge. Biometals 23:927–940. doi:10.1007/s10534-010-9329-x
Daud MK, Sun Y, Dawood M, Hayat Y, Variath MT, Wu YX, Raziuddin, Mishkat U, Salahuddin, Najeeb U, Zhu S (2009) Cadmium-induced functional and ultrastructural alterations in roots of two transgenic cotton cultivars. J Hazard Mater 161:463–473. doi:10.1016/j.jhazmat.2008.03.128
Djebali W, Zarrouk M, Brouquisse R, El Kahoui S, Limam F, Ghorbel MH, Chaibi W (2005) Ultrastructure and lipid alterations induced by cadmium in tomato (Lycopersicon esculentum) chloroplast membranes. Plant Biol 7:358–368. doi:10.1055/s-2005-837696
Dourado MN, Martins PF, Quecine MC, Piotto FA, Souza LA, Franco MR, Tezotto T, Azevedo RA (2013) Burkholderia sp. SCMS54 reduces cadmium toxicity and promotes growth in tomato. Ann Appl Biol 163:494–507. doi:10.1111/aab.12066
Dourado MN, Franco MR, Peters LP, Martins PF, Souza LA, Piotto FA, Azevedo RA (2015) Antioxidant enzymes activities of Burkholderia ssp. strains-oxidative responses to Ni toxicity. Environ Sci Pollut Res 22:19922–19932. doi:10.1007/s11356-015-5204-1
Fan SK, Fang XZ, Guan MY, Ye YQ, Lin XY, Du ST, Jin CW (2014) Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front Plant Sci 5:1–8. doi:10.3389/fpls.2014.00721
Fornazier RF, Ferreira RR, Vitoria AP, Molina SMG, Lea PJ, Azevedo RA (2002) Effects of cadmium on antioxidant enzyme activities in sugar cane. Biol Plant 45:91–97
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46. doi:10.1016/j.envexpbot.2012.04.006
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120. doi:10.1016/j.plantsci.2011.04.018
Gomes-Junior RA, Moldes CA, Delite FS, Pompeu GB, Gratão PL, Mazzafera P, Lea PJ, Azevedo RA (2006) Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere 65:1330–1337. doi:10.1016/j.chemosphere.2006.04.056
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494. doi:10.1071/FP05016
Gratão PL, Pompeu GB, Capaldi FR, Vitorello VA, Lea PJ, Azevedo RA (2008a) Antioxidant response of Nicotiana tabacum cv. Bright yellow 2 cells to cadmium and nickel stress. Plant Cell Tissue Org 94:73–83. doi:10.1007/s11240-008-9389-6
Gratão PL, Monteiro CC, Antunes AM, Peres LEP, Azevedo RA (2008b) Acquired tolerance of tomato (Lycopersicum esculentum cv. Micro-Tom) plants to cadmium-induced stress. Ann Appl Biol 153:321–333. doi:10.1111/j.1744-7348.2008.00299.x
Gratão PL, Monteiro CC, Rossi ML, Martinelli AP, Peres LEP, Medici LO, Lea PJ, Azevedo RA (2009) Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ Exp Bot 67:387–394. doi:10.1016/j.envexpbot.2009.06.017
Gratão PL, Monteiro CC, Carvalho RF, Tezotto T, Piotto FA, Peres LEP, Azevedo RA (2012) Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiol Biochem 56:79–96. doi:10.1016/j.plaphy2012.04.009
Gratão PL, Monteiro CC, Tezotto T, Carvalho RF, Alves LR, Peters LP, Azevedo RA (2015) Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals 28:803–816. doi:10.1007/s10534-015-9867-3
Guelfi A, Azevedo RA, Lea PJ, Molina SMG (2003) Growth inhibition of the filamentous fungus Aspergillus nidulans by cadmium: an antioxidant enzyme approach. J Gen Appl Microbiol 49:63–73
Han C, Shen HY, Ye J, Yang L, Liang S (2012) Effect of exogenous abscisic acid on tolerance of wheat seedlings to cadmium stress. Acta Botan Boreali-Occiden Sin 32:745–750, http://en.cnki.com.cn/Article_en/CJFDTotal-DNYX201204017.htm. Accessed 12 October 2015
Harrison E, Burbidge A, Okyere JP, Thompson AJ, Taylor IB (2011) Identification of the tomato ABA-deficient mutant sitiens as a member of the ABA-aldehyde oxidase gene family using genetic and genomic analysis. Plant Growth Regul 64:301–309. doi:10.1007/s10725-010-9550-1
Hartung W, Schraut D, Jiang F (2005) Physiology of abscisic acid (ABA) in roots under stress—a review of the relationship between root ABA and radial water and ABA flows. Aust J Agric Res 56:1253–1259. doi:10.1071/AR05065
Hermans C, Vuylsteke M, Coppens F, Craciun A, Inzé D, Verbruggen N (2010) Early transcription changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol 187:119–131. doi:10.1111/j.1469-8137.2010.03258.x
Hippler FWR, Boaretto RM, Quaggio JA, Azevedo RA, Mattos D (2015) Towards soil management with Zn and Mn: estimates of fertilization efficacy of citrus trees. Ann Appl Biol 166:484–495. doi:10.1111/aab.12197
Hoagland D, Arnon D (1950) The water-culture method for growing plants without soil. Circular. California Agricultural Berkeley: University of California, College of Agriculture, Agricultural Experiment Station, Berkeley
Hong JH, Seah SW, Xu J (2013) The root of ABA action in environmental stress response. Plant Cell Rep 32:971–983. doi:10.1007/s00299-013-1439-9
Hsu YT, Kao CH (2003) Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 26:867–874. doi:10.1046/j.1365-3040.2003.01018.x
Hu YF, Zhou G, Na XF, Yang L, Nan WB, Liu X, Zhang YQ, Li JL, Bi YR (2013) Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J Plant Physiol 170:965–975. doi:10.1016/j.jpiph.2013.02.008
Iannone MF, Groppa MD, Benavides MP (2015) Cadmium induces different biochemical responses in wild type and catalase-deficient tobacco plants. Environ Exp Bot 109:201–211. doi:10.1016/j.envexpbot.2014.07.008
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137–138
Kim TH (2014) Mechanism of ABA signal transduction: agricultural highlights for improving drought tolerance. J Plant Biol 57:1–8. doi:10.1007/s12374-014-0901-8
López-Chuken UJ, Young SD (2010) Modelling sulphate-enhanced cadmium uptake by Zea mays from nutrient solution under conditions of constant free Cd2+ ion activity. J Environ Sci 22:1080–1085. doi:10.1016/S1001-0742(09)60220-5
Lux A, Vaculík M, Martinka M, Lišková D, Kulkarni MG, Stirk WA, Van Staden J (2011) Cadmium induces hypodermal periderm formation in the roots of the monocotyledonous medicinal plant Merwilla plumbea. Ann Bot 107:285–292. doi:10.1093/aob/mcq240
Mäkelä P, Munns R, Colmer TD, Peltonen-Sainio P (2003) Growth of tomato and an ABA-deficient mutant (sitiens) under saline conditions. Physiol Plant 117:58–63. doi:10.1034/j.1399-3054.2003.1170107.x
Maksimović JD, Bogdanović J, Maksimović V, Nikolic M (2007) Silicon modulates the metabolism and utilization of phenolic compounds in cucumber (Cucumis sativus L.) grown at excess manganese. J Plant Nutr Soil Sci 170:739–744. doi:10.1002/jpln.200700101
Malavolta E, Vitti GC, Oliveira SA (1997) Assessment of nutritional status of plants: principles and applications, 2nd edn. Associação Brasileira para Pesquisa da Potassa e do Fosfato, Piracicaba, 319p
Mondal NK, Das C, Roy S, Datta JK, Banerjee A (2013) Effect of varying cadmium stress on chickpea (Cicer arietinum L.) seedlings: an ultrastructural study. Ann Environ Sci 7:59–70, http://hdl.handle.net/2047/d20018674
Monteiro CC, Carvalho RF, Gratão PL, Carvalho G, Tezotto T, Medici LO, Peres LEP, Azevedo RA (2011) Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environ Exp Bot 71:306–320. doi:10.1016/j.envexpbot.2010.12.020
Monteiro CC, Rolão MB, Franco MR, Peters LP, Cia MC, Capaldi FR, Carvalho RF, Gratão PL, Rossi ML, Martinelli AP, Peres LEP, Azevedo RA (2012) Biochemical and histological characterization of tomato mutants. An Acad Bras Cienc 84:573–585. doi:10.1590/S0001-37652012005000022
Moradi L, Ehsanzadeh P (2015) Effects of Cd on photosynthesis and growth of safflower (Carthamus tinctoruis L.) genotypes. Photosynthetica 53:506–518. doi:10.1007/s11099-015-0150-1
Nogueirol RC, Monteiro FA, Gratão PL, Borgo L, Azevedo RA (2015) Tropical soils with high aluminum concentrations cause oxidative stress in two tomato genotypes. Environ Monit Assess 187:73. doi:10.1007/s10661-015-4282-3
Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LSP (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202:35–49. doi:10.1111/nph.12613
Peters LP, Carvalho G, Martins PF, Dourado MN, Vilhena MB, Pileggi M, Azevedo RA (2014) Differential responses of the antioxidant system of ametryn and clomazone tolerant bacteria. Plos ONE 9:e112271. doi:10.1371/journal.pone.0112271
Piotto FA, Tulmann-Neto A, Franco MR, Boaretto LF, Azevedo RA (2014) Rapid screening for selection of heavy metal-tolerant plants. Crop Breed Appl Biotechnol 14:1–7. doi:10.1590/S1984-70332014000100001
Rendón MY, Gratão PL, Salva TJG, Azevedo RA, Bragagnolo N (2013) Antioxidant enzyme activity and hydrogen peroxide content during the drying of Arabica coffee beans. Eur Food Res Technol 236:753–758. doi:10.1007/s00217-013-1933-x
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212, http://www.ncbi.nlm.nih.gov/pubmed/13986422
Schellingen K, Van Der Straeten D, Remans T, Vangronsveld J, Keunen E, Cuypers A (2015) Ethylene signaling is mediating the early cadmium-induced oxidative challenge in Arabidopsis thaliana. Plant Sci 239:137–146. doi:10.1016/j.plantsci2015.07.015
Shekhawat GS, Verma K, Jana S, Singh K, Teotia P, Prasad A (2010) In vitro biochemical evaluation of cadmium tolerance mechanism in callus and seedlings of Brassica juncea. Protoplasma 239:31–38. doi:10.1007/s00709-009-0079-y
Shi WG, Li H, Liu TX, Polle A, Peng CM, Luo ZB (2015) Exogenous abscisic acid alleviates zinc uptake and accumulation in Populus × canescens exposed to excess zinc. Plant Cell Environ 38:207–223. doi:10.1111/pce.12434
Soares C, Sousa A, Pinto A, Azenha M, Teixeira J, Azevedo RA, Fidalgo F (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bot 122:115–125. doi:10.1016/j.envexpbot.2015.09.010
Stroiński A, Giźewska K, Zielezińska M (2013) Abscisic acid is required in transduction of cadmium signal to potato roots. Biol Plant 57:121–127. doi:10.1007/s10535-012-0135-x
Su Y, Liu J, Lu Z, Wang X, Zhang Z, Shi G (2014) Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ Exp Bot 97:40–48. doi:10.1016/j.envexpbot.2013.10001
Tal M (1966) Abnormal stomatal behavior in wilty mutants of tomato. Plant Physiol 41:1387–1394, http://www.plantphysiol.org/content/41/8/1387.full.pdf
Taylor IB, Lineorth RST, Al-Naieb R, Bowman WR, Marples BA (1988) The wilty tomato mutants flacca and sitiens are impaired in the oxidation of ABA-aldedyde to ABA. Plant Cell Environ 11:739–745
Thompson AJ, Thorne ET, Burbidge A, Jackson AC, Sharp RE, Taylor IB (2004) Complementation of notabilis, an abscisic acid-deficient mutant of tomato: importance of sequence context and utility of partial complementation. Plant Cell Environ 27:459–471. doi:10.1111/j.1365-3040.2003.01164.x
Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1 an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233. doi:10.1105/tpc.001388
Vitória AP, Lea PJ, Azevedo RA (2001) Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry 57:701–710
Vitória AP, Da Cunha M, Azevedo RA (2006) Ultrastructural changes of radish leaf exposed to cadmium. Environ Exp Bot 58:47–52. doi:10.1016/j.envexpbot.2005.06.014
Vitória AP, Rodriguez APM, da Cunha M, Lea PJ, Azevedo RA (2003) Structural changes in radish seedlings exposed to cadmium. Biol Plant 47:561–568. doi:10.1023/B:BIOP.0000041062.00539.7a
Wang Y, Wang Y, Kai W, Zhao B, Chen P, Sun L, Ji K, Li Q, Dai S, Sun Y, Wang Y, Pei Y, Leng P (2014) Transcriptional regulation of abscisic acid signal core components during cucumber seed germination and under Cu2+, Zn2+, NaCl and simulated acid rain stress. Plant Physiol Biochem 76:67–76. doi:10.1016/j.plaphy.2014.01.003
Wang J, Lin L, Luo L, Liao M, Lv X, Wang Z, Liang D, Xia H, Wang X, Lai Y, Tang Y (2016) The effects of abscisic acid (ABA) addition on cadmium accumulation of two ecotypes of Solanum photeinocarpum. Environ Monit Assess 188:1–8. doi:10.1007/s10661-016-5194-6
Wu Z, Zhao X, Sun X, Tan Q, Tang Y, Nie Z, Qu C, Chen Z, Hu C (2015) Antioxidant enzymes systems and the ascorbate-glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 138:526–536. doi:10.1016/j.chemosphere.2015.06.080
Zhu XF, Zheng C, Hu YT, Jiang T, Liu Y, Dong NY, Yang JL, Zheng SJ (2011) Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum. Plant Cell Environ 34:1055–1064. doi:10.1111/j.1365-3040.2011.02304.x
Acknowledgments
This work was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP—grants 2009/54676-0 and 2011/50982-9). R.A.A. and A.P.M. thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil) for the research fellowships. The authors also acknowledge “Centro de Microscopia e Imagem”, FOP/UNICAMP, and LBCM/CENA, for maintaining the electron and light microscopes, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests or conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Rights and permissions
About this article
Cite this article
Pompeu, G.B., Vilhena, M.B., Gratão, P.L. et al. Abscisic acid-deficient sit tomato mutant responses to cadmium-induced stress. Protoplasma 254, 771–783 (2017). https://doi.org/10.1007/s00709-016-0989-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-0989-4