Abstract
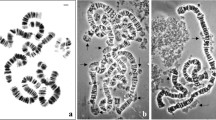
Multicolor 3D fluorescence in situ hybridization was used to study arrangement of rRNA genes in Calliphora erythrocephala nurse cell nuclei with different levels of polyteny. It has been shown that the rRNA genes are exclusively localized to chromosome 6, suggesting that chromosome 6 is the only C. erythrocephala chromosome responsible for nucleolar formation. We have also described changes in localization of ribosomal genes within the chromosome territory during polytenization, namely, that rDNA signals are detected in the peripheral region of chromosome territory starting from the stage of polytene chromosomes. In addition, it has emerged that large nucleolus associated with chromosome 6 starts to develop in the central nuclear region in the C. erythrocephala nurse cell nuclei at the stage of a primary reticular structure. The central position and nucleolar structure are retained at the stages when chromosome 6 occupies the central position, that is, at the stages of polytene and bloblike chromosomes. When the nucleus restores a reticular structure but at a higher polyteny level, the displacement of chromosome 6 to the nuclear periphery is accompanied by disruption of the large nucleolus into micronucleoli. The micronucleoli are distributed in the nuclear space retaining their association with the nucleolar-organizing regions of chromosome 6. Thus, our data suggest that the large-scale alterations in the organization of chromosome 6 and the nucleolus during polytenization are the correlated processes directly dependent on the rRNA gene activity. The earlier described dynamics of nucleolar-organizing chromosome territory and nucleolus in the nuclear space is likely to be associated with the change in the total expression activity of the nucleus, which complies with the hypothesis on the correlation between spatial nuclear organization and expression regulation of genetic material.





Similar content being viewed by others
References
Anan'ina TV, Vedernikov AE, Wasserlauf IE, Stegniy VN, Karamysheva TV, Rubtsov NB (2005) Visualization of chromosome territories in interphase nuclei of ovarian nurse cells in Calliphora erythrocephala Mg. (Diptera: Calliphoridae). Russ J Genet 41(10):1106–1112
Anan'ina TV, Kokhanenko AA, Khodzhanov AE, Stegniy VN (2007) Specific features in the structure of the egg tubes in the Calliphora erythrocephala (Mg.) (Diptera: Calliphoridae) ovaries. Vestn. Tomsk. Gos. Universiteta 297:175–180
Bier K (1957) Endomitose und politanie in den Nahrzellenkernen von Calliphora erythrocephala Meigen. Chromosoma 8:493–522
Boyes JW, Shewell GE (1975) Cytotaxonomy of Calliphoridae (Diptera). Genetica 45:435–488
Chubb JR, Boyle S, Perry P, Bickmore WA (2002) Chromatin motion is constrained by association with nuclear compartments in human cells. Curr Biol 12:439–445
Dekker J (2008) Gene regulation in the third dimension. Science 319:1793–1794
Fraser P, Bickmore W (2007) Nuclear organization of the genome and the potential for gene regulation. Nature 447:413–417
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33: 245–254
Kind J, van Steensel B (2010) Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol 22:320–325
Koehler D, Zakhartchenko V, Froenicke L, Stone G, Stanyon R, Wolf E, Cremer T, Brero A (2009) Changes of higher order chromatin arrangements during major genome activation in bovine preimplantation embryos. Exp Cell Res 315:2053–2063
Kokhanenko AA, Anan'ina TV, Stegniy VN (2013) The changes in chromosome 6 spatial organization during chromatin polytenization in the Calliphora erythrocephala Mg. (Diptera: Calliphoridae) nurse cells. Protoplasma 250(1):141–149
Kokhanenko AA, Anan'ina TV, Stegniy VN (2010) Intranuclear dynamics of chromosome 6 in nurse cells of Calliphora erythrocephala Mg. (Diptera: Calliphoridae). Russ J Genet 46(9):1045–1047
Kulichkov VA, Zhimulev IF (1976) Analysis of genome spatial organization Drosophila melanogaster on the base of data by ectopic conjugation of polytene chromosomes. Genetica 12(5):81–89
Kumar V, Cornel AJ, Mukabayire O (1997) In situ hybridization to Anopheles polytene chromosomes. Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman and Hall, London, p 337–345
Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T (2007) Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet 8:104–115
Lichter P, Ledbetter SA, Ledbetter DH, Ward DC (1990) Fluorescence in situ hybridization with Alu and L1 polymerase chain reaction probes for rapid characterization of human chromosomes in hybrid cell lines. Proc Natl Acad Sci USA 87:6634–6638
Martou G, De Boni U (2000) Nuclear topology of murine, cerebellar Purkinje neurons: changes as a function of development. Exp Cell Res 256:131–139
Mayer R, Brero, von Hase J, Schroeder T, Cremer T, Dietzel S (2005) Common themes and cell type specific variations of higher order chromatin arrangements in the mouse. BMC Cell Biol 6:44. doi:10.1186/1471-2121-6-44
Molnar M, Kleckner N (2008) Examination of interchromosomal interactions in vegetatively growing diploid Schizosaccharomyces pombe cells by Cre/loxP site-specific recombination. Genetics 178:99–112
Mukherjee K, Storici F (2012) A mechanism of gene amplification driven by small DNA fragments. PLoS Genet. doi:10.1371/journal.pgen.1003119
Naumova NM, Olenkina OM, Gvozdev VA (2003) Inactivation of reporter genes by cloned heterochromatic repeats of Drosophila melanogaster is accompanied by chromatin compaction. Russ J Genet 39(5):559–563
Neusser M, Schubel V, Koch A, Cremer T, Müller S (2007) Evolutionarily conserved, cell type and species-specific higher order chromatin arrangements in interphase nuclei of primates. Chromosoma 116:307–320
Ribbert D (1979) Chromosomes and puffing in experimentally induced polytene chromosomes of Calliphora erythrocephala. Chromosoma (Berl) 74:269–298
Rubtsov NB, Alekseenko AA, Belyaeva ES (1999) Microcloning and characteristics of DNA from regions of the centromeric heterochromatin of Drosophila melanogaster polytene chromosomes. Rus J Genetics 1:55–61
Schneider R, Grosschedl R (2007) Dynamics and interplay of nuclear architecture, genome organization, and gene expression. Genes Dev 21:3027–3043
Solovei I, Kreysing M, Lanctot C, Kosem S, Peichl L, Cremer T, Guck J, Joffe B (2009) Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137:356–368
Stark GR (1993) Regulation and mechanisms of mammalian gene amplification. Adv Cancer Res 61:87–113
Stegniy VN (1979) Reorganization structure of interphase nucleus in onto- and phylogenesis of malaria mosquito. Referents of AS USSR 249(5):1231
Stegniy VN (2006) Evolutionary significance of chromosome architecture for epigenetic control of eukaryote development and phylogeny. Russ J Genet 42(9):1011–1018
Vasserlauf IE, Anan'ina TV, Unger MF, Karamysheva TV, Mel'nikova NN, Rubtsov NB, Stegniy VN (2003) Organization and differential staining of the chromosomes of Calliphora erythrocephala (Diptera: Calliphoridae) nurse cell endomitotic nuclei. Genetika 39(9):1193–1202
Volpi EV, Chevret E, Jones T, Vatcheva R, Williamson J, Beck S, Campbell RD, Goldsworthy M, Powis SH, Ragoussis J, Trowsdale J, Sheer D (2000) Large-scale chromatin organization of the major histocompatibility complex and other regions of human chromosome 6 and its response to interferon in interphase nuclei. J Cell Sci 113(Pt 9):1565–76
Whiting MF, Carpenter JC, Wheeler QD, Wheeler WC (1997) The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Syst Biol 46(1):1–68
Zhimulev IF (1992) Polytene chromosomes: morphology and structure. Nauka, Novosibirsk
Zink D, Amaral MD, Englmann A, Lang S, Clarke LA et al (2004) Transcription-dependent spatial arrangements of CFTR and adjacent genes in human cell nuclei. J Cell Biol 166:815–825
Acknowledgments
We thank Gleb Artemov for providing protocol of fluorescence in situ hybridization (FISH). The work was supported by the scholarship of President RF (SP-1037.2013.4) and supported, in part, by the Federal Grant Targeted No. 14.B37.21.0114 and by state assignment No. 4.5612.2011 (Ministry of Education and Science RF).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Jan Raoul De Mey
Rights and permissions
About this article
Cite this article
Kokhanenko, A., Anan’ina, T. & Stegniy, V. Localization of rRNA genes in the nuclear space of Calliphora erythrocephala Mg. nurse cells during polytenization. Protoplasma 251, 93–101 (2014). https://doi.org/10.1007/s00709-013-0529-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0529-4