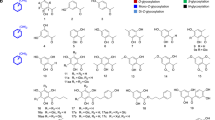
Abstract
A practical and scalable synthesis of C-glycosides identified as highly potent sodium-dependent glucose transporter 2 (SGLT2) inhibitors is described. Highlights of the synthetic process are a concise, ten-step synthesis of a structurally complex active pharmaceutical ingredient from a known intermediate via a selective and quantitative addition of an aryl species to the Weinreb amide, and the isomers of undesired ortho-products were avoided during the preparation. The chemistry developed has been applied to prepare SGLT2 inhibitors without recourse to chromatography.
Graphical abstract

Similar content being viewed by others
References
Mathis D, Vence L, Benoist C (2001) Nature 414:792
Cerasi E, Kaiser N, Leibowitz G (2000) Diabetes Metab 26:13
Wright EM, Turk E (2004) Pfluegers Arch 447:510
Asano T, Ogihara T, Katagiri H (2004) Curr Med Chem 11:2717
Ehrenkranz RRL, Lewis NG, Kahn C, Roth J (2005) Diabetes Metab Res Rev 21:31
Wells R, Pajor A, Kanai Y (1992) Am J Physiol 263:459
Anthony L (2005) Expert Opin Ther Patents 15:1531
Prous JR, Serradell N, Munoz R (2013) Pyrano[3,2-c][2]benzopyran-6(2H)-one derivatives and uses thereof. WO Patent 2,013,045,495, Apr 4, 2013; (2013) Chem Abstr 158:504416
Binhua L, Baihua X (2009) Bioorg Med Chem Lett 19:6877
Binhua L, Baihua X (2010) Bioorg Med Chem Lett 18:4422
Yoshihito O, Tsutomu S, Takamitsu K (2012) J Med Chem 55:7828
Binhua Lv, Yan F, Jiajia D, Min X, Baihua X (2010) Chem Med Chem 5:827
Tsutomu S, Kiyofumi H (2008) Substituted spiroketal derivative and use thereof as drug for treating diabetes. WO Patent 2,008,013,280, Jan 18, 2008; (2008) Chem Abstr 148:215271
Chen YW, Feng Y, Xu BH, Lv BH (2008) Preparation of benzylic glycoside derivatives and their inhibitory effect on sodium-dependent glucose cotransporter SGLT. US Patent 20,080,242,596, Oct 2, 2008; (2008) Chem Abstr 149:378967
Chen Y, Feng, Y, Xu B (2007) Preparation of spiro glycosides as glucose transport inhibitors. US Patent 20,070,275,907, Nov 29, 2007; (2007) Chem Abstr 147:542063
Christian B, Gesche R, Michele Z (2009) J Org Chem 74:8779
Liu YH, Ting MF, Ou CY (2013) Chin Chem Lett 24:131
Acknowledgements
This work was financially supported by Jiangsu Province Natural Science Fund (20131414) and by the National Natural Science Foundation of China (51403104).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Fu, T., Chen, Z. et al. A new and improved process for C-aryl glucoside SGLT2 inhibitors. Monatsh Chem 146, 1715–1721 (2015). https://doi.org/10.1007/s00706-015-1458-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1458-z