Abstract
Despite numerous efforts, we still do not have prophylactic vaccines for many clinically relevant viruses, such as HIV, hepatitis C virus, Zika virus, and respiratory syncytial virus. Several factors have contributed to the current lack of effective vaccines, including the high rate of viral mutation, low immunogenicity of recombinant viral antigens, instability of viral antigenic proteins administered in vivo, sophisticated mechanisms of viral immune evasion, and inefficient induction of mucosal immunity by vaccine models studied to date. Some of these obstacles could be partially overcome by the use of vaccine adjuvants. Nanoparticles have been intensively investigated as vaccine adjuvants because they possess chemical and structural properties that improve immunogenicity. The use of nanotechnology in the construction of immunization systems has developed into the field of viral nanovaccinology. The purpose of this paper is to review and correlate recent discoveries concerning nanoparticles and specific properties that contribute to the immunogenicity of viral nanoparticle vaccines, bio-nano interaction, design of nanoparticle vaccines for clinically relevant viruses, and future prospects for viral nanoparticle vaccination.
Similar content being viewed by others
Introduction
Prevention of viral infection is the best – perhaps the only – way to reduce public health problems resulting from viral diseases. There have been historic successes in the eradication or prevention of viral diseases such as smallpox, polio, hepatitis A, and papilloma by vaccines [1,2,3,4]. New antigen preparations, strategies for vaccination (including weakly transmissible vaccines), and sequential programs of public immunization have contributed to improvements in the efficacy of viral vaccines, among which the emergence of nanotechnology holds great promise [5,6,7]. Nanotechnology has been applied to vaccine development and optimization using several sophisticated nanoparticle (NP) systems to improve antigen delivery and efficient induction of immunity [8, 9]. One of the main advantages of using NPs in vaccines is that they can deliver peptides or proteins to specific tissues and help the cells to generate a stronger immune response [8,9,10]. In addition, NPs protect the applied antigen from enzymatic degradation, which is particularly relevant for mucosal vaccination [11]. Moreover, NPs are biocompatible, biodegradable, and relatively easy to produce [12,13,14,15]. These properties of NPs improve the bioavailability of the antigen in vivo and influence the innate and adaptive immune response, including the generation of memory cells, which are essential for a vaccine’s success [16,17,18,19,20,21].
There has been an increase in research in the field of viral NP vaccines, and therefore, the aim of this review is to discuss the main ways in which viral NP vaccines affect the immune response to present some NP vaccine models that have been studied for clinically relevant viruses, and to provide future perspectives for the development and use of these vaccines.
Nanoparticle models used for delivery of viral antigens
The first point to consider while choosing an NP model to develop a vaccine is the antigen encapsulation and delivery of the system. The most frequently used models of NP vaccines that have been tested for delivery of viral antigens are liposomes – cationic vesicles entrapping an antigen to transfect cells [22]; PEG-PLA (polyethylene glycol-polylactic acid] and PLGA [poly(D,L-lactic-co-glycolic acid) — biodegradable polymers used to encapsulate vaccine antigens and adjuvants, which are frequently used for antigen protection and long-term delivery [23]; interbilayer-crosslinked multilamellar vesicles (ICMVs) — nanovesicles constructed to stabilize liposomes, reduce their toxicity, and increase the time of antigen release in vivo [24]; chitosan [(1, 4)-2-amino-2-deoxy-D-glucan] — natural polymer with biodegradable, biocompatible, adhesive, and non-toxic properties, which is specially used for mucosal applications [25]; protein cages — non-infectious particles constructed from viral structural proteins by recombinant technology to produce virus-like particles (VLPs); virosomes — lipid bilayers conjugated with viral glycoproteins specific for target-cell receptors used to facilitate antigen delivery into cytoplasm [26], and ISCOMs — immune-stimulating complexes with phospholipids and saponins [27]. A schematic diagram of each of these NP vaccine models, which are already being used for viral immunization, is presented in Table 1.
Nanoparticle properties involved in the induction of the immunological response
All immunological benefits of NPs result from a combination of the following properties: 1) their capacity to mimic the virus in terms of size and structure without requiring an actual infection, 2) their ability to deliver viral antigens in a repetitive manner, 3) the ability to activate dendritic cells (DCs) and antigen cross-presentation, and 4) their ability to activate follicular B cells efficiently and induce a humoral immune response. A discussion of each of these points is presented below.
Pathogen mimicry and virus-like particles (VLPs)
The main nanosystem already being applied for viral vaccination is the VLP system, sometimes called protein cages. VLPs are self-assembling non-infectious NPs that lack genetic material but maintain structured capsid proteins [28] (Fig. 1A). VLPs present repetitive immunodominant epitopes, which optimize the activation of a specific immune response consisting of humoral as well as cellular immunity [29]. The first VLP vaccine licensed for human use was the hepatitis B surface antigen (HBsAg). This NP is produced in yeast, is approximately 22 nm in diameter, and induces protection by high-affinity IgG antibodies, as well as CD4 and CD8 T cells [30]. Recently, Wang et al. tested the VLPs containing hepatitis B core antigen (HBcAg) by creating CpG-Au@HBc VLP as an immunostimulatory nanocomposite. This NP was tested in mice, and an enhanced HBc-specific immune response through IgG production and an increase in the T cell response and IFN-γ and IL-2 secretion were observed [31]. Another VLP applied in humans is the vaccine against human papillomavirus (HPV). This vaccine is an NP with a diameter of 30 to 60 nm and is composed only of viral capsid protein [32]. VLPs are very promising systems, but it is hard to select and improve the immunogenicity of candidate epitopes to produce a protective vaccine using this model. Recent studies in pigs using VLPs from porcine reproductive and respiratory syndrome virus (PRRSV) showed that PRRSV VLPs induced an anamnestic response with specific IgG and IFN-γ, but this did not provide efficient protection against in vivo challenge [33]. Thus, even the presence of immunodominant epitopes does not ensure that a VLP vaccine will be effective.
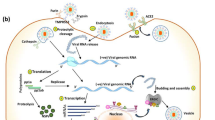
Morphological and functional aspects of NPs that resemble mechanism of viral immune activation. A) Virion and virus-like particle. B) Releasing antigens loaded into NPs. C) Activation of costimulatory receptor on antigen-presenting cells by PRR ligands loaded together with viral antigens into NPs. D) Activation of B cell, plasma cell, and antibody production through antigen loaded in an NP vaccine
Loading and delivery of viral molecules
The two main obstacles to using immunogenic viral peptides for vaccination are, on one hand, that they are easily degraded at the site of injection and, on the other hand, that they are too small to be phagocytized by antigen-presenting cells (APCs). Furthermore, unprotected peptides are instable when used for mucosal vaccination, which is the more conducive route for immunization against mucosally transmissible pathogens. In this regard, the use of NPs for antigen delivery is an alternative that would help to overcome the problem of peptide instability and make mucosal delivery feasible. Furthermore, NPs can deliver peptides to specific sites and carry antigens and adjuvants in the same particle. Figure 1B shows the basic design of an NP for loading and delivering antigen. In a study conducted by Dhakal and colleagues, an NP loaded with a cocktail of conserved peptides containing T and B cell epitopes from human H1N1 influenza A virus and M2e-PP of swine influenza virus H1N1 entrapped in PLGA-NPs was used to vaccinate pigs by the intranasal route and was found to induce efficient protection after challenge [34]. The benefits of NPs for the delivery of antigens were also observed with an NP vaccine prepared with HTLV env13 and env23 immunodominant peptides. These particles were used to inoculate mice by the nasal or subcutaneous route, and high titers of serum IgG, IgG1 and IgG2a were observed 30 days post-immunization [35].
In addition to providing protection, NPs carrying viral antigens can improve antigen transport from the site of injection to the lymphatic system [36,37,38]. The entry of macromolecules into lymphatic vessels depends on their size, with molecules between 20 and 200 nm easily crossing lymphatic gaps [39]. It is interesting that small polymeric NPs (25 nm) can be retained in the draining lymph nodes of mice for at least 120 h after injection, and they colocalize with macrophages and dendritic cells [40]. Thus, the size of the NP vaccine influences the kinetics of distribution within the secondary lymphoid system in mice. Together with their easy delivery through the lymphatic system, another advantage of NPs is their effect on DC maturation. One of the first studies that proposed the use of NPs as pathogen-mimicking systems showed that these nanostructures induce the maturation of mouse DCs by increasing the expression of cellular MHC class II, CD80 and CD40 as well as stimulating the production of high levels of proinflammatory cytokines and secretion of chemokines, including IL-6, IL-12, TNF-α, MIP-1α (CCL3) and RANTES (CCL5) (Th1 polarization) [38]. In vivo studies in mice have shown that after intradermal injection, NPs are taken up by DCs that are drained to the local lymph nodes, and 48 h after subcutaneous injection, the nanocarrier can still be detected in the lymphoid organ [47]. The in vitro and in vivo improvements in the immune response result, in part from the protection and delivery provided by the nanosystem to the antigen that it is carrying.
Activation of dendritic cells and cross-presentation
DCs express pattern-recognition receptors (PRRs), such as Toll-like receptors (TRLs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs), which are associated with recognition of pathogen-associated molecular patterns (PAMPs), DC maturation, and the expression of inflammatory cytokines [41, 42]. TLR3 and TRL7/8 are endosomal receptors that bind dsRNA and ssRNA, respectively, while TLR9 can be trigged by dsDNA and RIG-mediated sensing of viral RNA. The activation of any of these intracellular receptors in infected cells induces the production of type I IFN, an inflammatory cytokine that is responsible for establishing an antiviral state [43, 44]. Adding PRR agonists as adjuvants in conjunction with antigens in a nanostructured system enhances the immune response in mice via activation of different PRRs [45, 46]. Figure 1C shows the possible PRRs ligands loaded into a NP and the target DC receptor. Among the PRR ligands that can be loaded into NPs to generate a viral vaccine, the one that has been investigated most extensively is the TLR9 ligand CpG [47,48,49]. One study using mice demonstrated that the density of CpG, associated with the particle size, controls DC signaling dynamics. NPs loaded with a high density of CpG induced DCs to produce more IL-12p70 via NFκβ/TLR9-IRAK4, resulting in Th1 polarization via IFN-γ production by CD4+ T cells. In contrast, NPs loaded with CpG at low density induced STAT3 signaling in DCs, leading to the inhibition of NFκβ and Th2 polarization [50].
The most peculiar mechanism observed in DCs treated with NPs loaded with antigens is cross-presentation (the process in which an exogenous antigen uses the MHC I pathway). The intracellular routes for cross-presentation involving nanostructures are still under investigation, but the phenomenon has been observed and characterized for some models of NPs in mice. Mukai et al. showed that exogenous antigen encapsulated into a γ-PGA NP poly(γ-glutamic acid-based NP) enter the cells by phagocytosis and/or macropinocytosis and passes through the MHC I pathway. The mechanism involves the induction of endoplasmic reticulum (ER) vesicle-endosome fusion by the NP, after which the confined antigen is retrotranslocated via the ER translocon Sec61 (endoplasmic reticulum membrane protein translocator) to the cytosol [51]. Cross-presentation and citotoxic T cell induction via endosomal entry can also be induced by protein-cage NPs. In this model, CpG and MHC I OVA peptide (SIINFEKL) are conjugated through an acid-labile hydrazine bond to an E2 (subunit of pyruvate dehydrogenase) protein cage. After DC endocytosis, the hydrazine bond is broken in the acidic environment of the endosome, and the SIINFEKL peptide is processed or displayed on MHC I (i.e., cross-presented) [52].
B cell activation and humoral response
The laboratory parameter that most frequently correlates with protection after vaccination against viruses and other microorganisms is the antibody titer, and NP vaccine models have been shown to induce high titers of serum antibodies [53,54,55,56]. Small particles (< 200 nm) can freely enter into the initial lymphatic vessel, migrate to the lymph node in a few hours, and reach and activate follicular B cells, whereas particles larger than 200 nm require DCs to transport them to follicular areas of the lymph nodes [57]. A B-cell response is normally induced by recognition of highly repetitive structures, and in this respect, the use of NPs is very beneficial, since they can be constructed to carry a large number of repetitive viral peptides or proteins [60]. Upon cross-linking of the B cell receptor (BCR) with monovalent antigen, the cell receives a signal. If several receptors are engaged by several monovalent Ag signals (like a cluster), it crosses a threshold to initiate activation [58]. An NP vaccine can therefore provide a high local concentration of a specific antigenic peptide for efficient BCR signaling, thus improving the B cell response (Fig. 1D). The positive effect of using NPs on the humoral response has been demonstrated by Irvine’s group. They constructed ICMVs conjugated with a Plasmodium vivax protein (VMP001) and used it to vaccinate mice. A strong humoral response was not observed until 400 days after immunization, and this response was associated with the presence of NPs in the follicular areas of the lymph nodes and progression to germinal center (GC) formation. NPs promoted robust GC formation at low doses of antigen, whereas no GC induction occurred when soluble protein was used. In parallel, NP immunization enhanced the expansion of antigen-specific follicular helper T cells when compared to vaccination with soluble VMP001 or alum [59].
Bionano-interactions: similarities between nanoparticle-cell and virus-cell interactions
After analysis of recent studies in NPs, we can suggests that the similarities between nanoparticle-cell interactions and virus-cell interactions are not intrinsic to the NP itself, but result from a combination of parameters, including the method of preparation, the type of molecule loaded, and the combination of specific ligands on the surface of the NP. Figure 2 illustrates the main routes shared by viruses and NPs for cell internalization.
Comparison of cell entry of viruses and nanoparticles. Virus entry: A)Virus binding to a specific receptor on cell surface; B) virus internalized by a clathrin-mediated process; C) virus internalized by a caveolin-mediated process; D) virus moves through the cytoplasm using microtubules and kinesin as a receptor. Nanoparticle entry: E) Nanoparticles that are similar in size to the virus can be internalized by cells. F) Positively charged nanoparticles are more attracted than negatively charged nanoparticles to the cell membrane. G) Adherent nanoparticles bind to cell membranes more easily than nonadherent ones. H) Contact between a nanoparticle and the cell membrane can induce the release of hyppos (Damp). I) Like viruses, NPs can use clathrin for cell internalization. J) Like viruses, nanoparticles can use caveolin for cell internalization K) Like viruses, nanoparticles can disrupt microtubules
Three cellular mechanisms can be involved in internalization of NPs: clathrin-, caveolae-, and microtubule-dependent, all of which are micropinocytotic pathways. The choice of mechanism depends on the size and charge of the NP (Fig. 2E and F). Epithelial cells use caveolae and microtubules to transport NPs of any size, while endothelial cells use caveolae and/or microtubules for larger NPs and caveolae/clathrin for smaller NPs [60]. Small particles can use multiple different micropinocytotic processes to reach intracellular compartments, while larger NPs are more limited to caveolae [61]. The mechanisms of cell entry used by NPs have already been thoroughly described for several viruses in murine and human cells models. For instance, during virus entry, a loaded NP can disrupt the cytoskeleton and microtubules, leading to the activation of NFκB [62]. Similarly, NPs can be transported by microtubules, which could activate the expression of NFκB and initiate a local microinflammatory process. This process may contribute to the adjuvanticity and antiviral immunity induced by a NP vaccine (Fig. 2D and K). In respiratory syncytial virus infection, the mobilization of clathrin and actin are important for IFN-γ release [63], while dengue virus type 3 (DENV-3) entry into host cells depends on the usage of clathrin-mediated endocytosis [64, 65]. The entry of hepatitis C virus (HCV) into hepatocytes also depends on clathrin-mediated endocytosis, while the progression of herpes simplex virus 1 (HSV-1) infection is facilitated by caveolin-1 [66, 67].
Although NPs do not bind to specific receptors, as the virus does, internalization can still be achieved through the positive or negative surface charge interactions (Fig. 2A and F). Considering the negative charge of the cell membrane, we can deduce that NPs with a positive charge are more likely to be taken up by cells than those with a negative charge. Indeed, some studies have indicated that the rate of uptake of positive NPs is higher than that of negative NPs [61, 68,69,70].
Once an NP interacts with a cell, there are other factors that contribute to the stability of the NP-cell interaction and facilitate uptake into the cell, such as the adhesivity of the NP (Fig. 2G). Bernkop-Schnürch and Greimel investigated the adhesivity of chitosan, a linear polysaccharide composed of randomly distributed β-(1→4)-linked D-glucosamine (deacetylated) and N-acetyl-D-glucosamine (acetylated) units, and showed that the mucoadhesive property of this polymeric matrix is potentially useful for mucosal antigen delivery [71]. Specific ligands are frequently chemically bonded to the surface of the NPs to promote strong, robust and specific nanoparticle-cell interactions. NPs for vaccine applications have already been developed using gold [72], CpG oligodeoxynucleotides (CpG ODN) [73], and silica, such as mesoporous silica [74], amorphous silica [75], and silica nanorattles [76].
Seong and Matzinger have suggested that hydrophobicity per se can be considered a danger-associated molecular pattern (DAMP). They propose that during any necrotic cell disruption or protein denaturation, some hydrophobic cellular materials, designated “hyppos”, are released and interact with receptors on the cell surface. These “hyppos” work as a DAMP and lead to the activation of the neighboring cell [77]. Viruses and NPs can induce formation of “hyppos” (Fig. 2H). To investigate the contribution of the hydrophobicity of NPs to the immune response, Moyano et al. developed eight gold NPs with increasing levels of hydrophobicity (NP1 to NP8). The authors demonstrated a direct correlation between the level of hydrophobicity and inflammatory potential. They showed that the level of expression of TNF-α RNA induced by NP1 (least hydrophobic) was lower than that induced by NP8 (most hydrophobic) [78].
Nanoparticle vaccine candidates for use against clinically relevant viral diseases
Dengue virus
An efficient vaccine against DENV should induce simultaneous immunity to all four serotypes of the virus, and a subunit vaccine containing parts of all serotypes is an option. Studies in mouse models using the major DENV immunogen, the E protein, in NPs has produced promising results. Metz et al. produced a PLGA NP with recombinant DENV-2 E protein adsorbed and used this to immunize mice. The adsorption of the E protein to PLGA increased the anti-E IgG titer and improved the neutralizing capacity of the antibodies, when compared to free E protein. These results were attributed to the retention of the DENV NP in lymph nodes, which increased the bioavailability of the antigen [79]. Another NP vaccine tested for DENV was produced using chitosan with UV-inactivated DENV-2 and Mycobacterium bovis Bacillus Calmette-Guerin cell wall components as an adjuvant. This nanosystem was tested in human DCs and showed a higher rate of phagocytosis for DENV chitosan NP than for soluble UV-inactivated dengue virus. After phagocytosis, human DCs showed significant upregulation of the cell markers CD80, CD86 and MHCII (HLA-DR). The benefits of using a chitosan NP vaccine against dengue was observed in intraperitoneal immunization of mice with UV-inactivated DENV-2 loaded into an NP containing chitosan/Mycobacterium bovis Bacillus Calmette-Guerin cell wall components. Vaccinated mice exhibited upregulated expression of IFN-γ, IL-2, IL-5, IL-12p40, IL-12p70, and IL-17, an increased frequency of CD4+IFN+ T cells, and higher levels of IgG antibodies specific for DENV in a dose-dependent manner [80, 81]. An NP vaccine based on chitosan was also tested for delivery of E protein from DENV-3 as an alternative nasal vaccine. The formulation of DENV-3 E-protein loaded into a chitosan NP was taken up more efficiently by nasal epithelial cells than free E protein and resulted in increased secretion of IL-1β, IL-6, and TNF-α [82].
Hepatitis B virus
The efficacy of the prophylactic vaccine against hepatitis B virus (HBV) is 90% to 95%. However, the World Health Organization (WHO) estimates that 257 million people are living with HBV [83]. The most serious problems related to chronic HBV infection are cirrhosis and hepatocellular carcinoma [84]. The current hepatitis B vaccine is a VLP made with HBsAg adsorbed to aluminum as an adjuvant. This combination is effective, but some toxic effects have been attributed to the aluminum (inflammatory conditions, autoimmunity, damage to the nervous system). This adjuvant also favors Th2 polarization and does not induce efficient mucosal protection [85,86,87]. Preliminary studies have demonstrated that application of nanotechnology can optimize the HBV vaccine delivery system, induce Th1 polarization, and reduce the risk of toxic effects.
An NP vaccine that was prepared by encapsulation of HBsAg into PEG-PLGA and used to immunize mice was found to produce a significant increase in serum anti-HBS antibodies. The anti-HBS antibodies were detected in mice two weeks after immunization with NP/HBsAg, and the levels were greater than 100 mIU/mL, reaching 800 mIU/mL after five weeks, whereas mice receiving non-encapsulated recombinant HBsAg reached a titer of only 95 mIU/mL at the same final week [88]. Protective antibody levels obtained with the conventional vaccine are approximately 10 mIU/mL [89]. Another NP model using PLGA absorbed with HBsAg and the TLR9 agonist CpG, when injected intramuscularly into mice, induced local expression of IL-1β and IL-6 and the chemokines CCL2, CXCL1 and CXCL9, followed by a significant HBsAg-specific response mediated by effector CD8 T cells and antibody-secreting B cells in the spleen [92].
Mucosal HBV vaccines are very desirable because they could increase patient compliance and induce IgA antibodies at the site of infection, which is important for preventing sexual transmission and improving the efficiency of mass immunization programs. In this field, promising results have been with NP vaccine models. Sahu and Pandey have investigated using the colonic mucosa as a site of delivery for a vaccine formulated as a liposome HBsAg/TLR4-ligand, and the efficiency of this nanosystem was clearly demonstrated by the high titers of local anti-HBS IgA compared to non-encapsulated conventional HBsAg vaccine [90]. The benefits of the mucosal HBsAg NP were also seen when this viral subunit was entrapped in polycaprolactone NP (PLC HBsAg NP) and administered orally to mice. The results indicated that six hours after oral administration, the PLC HBsAg NP reached different organs (liver, small intestine, lungs and inguinal lymph node), and significant levels anti-HBsAg IgA were detected in saliva eight weeks after oral treatment [91]. High titers of anti-HBsAg IgA were observed at mucosal sites after subcutaneous immunization with HBsAg-loaded PCL/chitosan and boosting by the intranasal route. Interestingly, again in this study, the anti-HBsAg titers were higher when using NPs than when using free antigen [92].
Only 5 to 10% of adults with recent infection develop chronic hepatitis, however, around 90% of neonates develop a chronic infection due to mother-to-child transmission. The reason for this is that the neonatal immune system is Th2 polarized and tolerogenic, with low levels of IL-12 production by DCs [98, 99]. Regarding NPs for delivery of antigens, a recent study has shown that combining HBsAg and the adjuvant monophosphoryl lipid A (MPLA) in the same NP influences the maturation of umbilical cord DCs from neonates in vitro. These cells become IL-12 producers and stimulate Ag-specific T cell proliferation and IFN-γ production, which is important for the antiviral response. Again, the conventional HBsAg VLP was found to be ineffective compared to the NP system [93].
Human immunodeficiency virus
The challenge in developing a successful vaccine against HIV is to produce a system capable of inducing neutralizing antibodies against diverse strains of the virus and also minimize the effects of mutations on the immunity already produced [94,95,96,97]. One of the promising immunogens for HIV vaccines is BG505 SOSIP.664, a gp120/gp41 self-assembled trimer that induces broadly neutralizing antibodies (bNAs) against autologous and resistant virus strains in mice [98]. This structure constitutes a VLP, induces high titers of neutralizing antibodies in mice and rabbits [99], and has been tested in NP models. The optimization and application of BG505 SOSIP.664 trimer for HIV vaccination depends on an effective system to deliver the vaccine to APCs and to efficiently stimulate CD4 and CD8 T cells. Advanced, and very elegant, studies to better characterize bNAs induced by this trimer in self-assembling NPs have demonstrated that this system provides an efficient link between the B cell receptor and the HIV cognate antigen, leading to B cell stimulation and development [100]. The BG505 SOSIP.664 trimer linked to ferritin NP induced stronger antibody response in mice and rabbits when compared to soluble antigen [99]. An increase in bNAs after administration of HIV env antigens adsorbed onto polystyrene NPs was accompanied by the production of antibody-secreting cells in immunized mice [101]. The benefit of using HIV Env trimers linked to synthetic NPs for B cell activation in vitro and in vivo was also observed using liposomes, since NPs with Env trimers covalently linked to the outer surface stabilize the antigen more efficiently than soluble trimers, leading to better cross-linking with the B cell receptor and inducing Ca2+ influx. This results in an increase in B cell activation, the number of germinal-center B cells, and high serum titers of anti-HIV IgG [102].
Significant titers of anti-HIV IgG antibodies were obtained using polymeric NPs carrying a combination of HIVp24-Nef fusion peptide and the bacterial flagellin from Pseudomonas aeruginosa as a TLR5 agonist. However, in this study, use of the NPs as a delivery system reduced the dose of HIV antigen and flagellin (adjuvant) needed to achieve a cellular response (proliferation and IFN-γ secretion) similar to what is observed with Freund´s adjuvant [103]. The advantages of nanoencapsulation of HIV Env trimers over soluble antigen has also been demonstrated with ICMVs. The gp140 anchored in the ICMV stabilizes the trimeric form of the antigen, maintaining conformational epitopes for B cell induction and leading to a better humoral response in a mouse model than is achieved using unanchored antigens [104]. The application of NPs for delivery of HIV immunogens was recently reviewed by Aikins et al. These authors describe recent studies using vectors, self-assembling “virus-like” NPs, synthetic NPs, and siRNA-based nanotherapeutics as strategies to improve HIV vaccination and therapy [105].
Influenza virus
Nowadays, the most efficient way to induce protection against influenza virus is vaccination with trivalent inactivated flu vaccine (TIV). However, the TIV formulation available is injected intramuscularly and generates systemic immunity but lacks the same efficiency for local immunity [106, 107]. Full protection against influenza virus in the mucosal respiratory tract is desirable. Indeed, there have been more studies using NP vaccines against influenza virus, than any other virus and the reason for this is that NPs have characteristics that make them suitable for mucosal immunization, inducing the type of immunity that is effective against respiratory viruses.
One of the simplest NP vaccines tested against influenza virus is a mixture of VLPs with TIV used to immunize mice. The VLP/TIV combination was found to be superior to free TIV for induction of anti-influenza immunity based on IgG, IgG2a and IgA titers in serum and bronchoalveolar lavage samples, especially after intranasal immunization [108]. Successful nasal mucosa immunization with NPs was also achieved in rabbits immunized with influenza whole virus (WV) incorporated into the mucoadhesive carrier chitosan. Ninety days after immunization with chitosan/WV plus CpG, significant levels of hemagglutinin inhibition antibody was detected in the serum, accompanied by local anti-influenza-virus IgA production, systemic IL-2, and IFN-γ production, the levels of which were much lower in rabbits immunized with WV alone [109]. An NP vaccine produced for H5N2 using H5 hemagglutinin trimer encapsulated in polyanhydride NCs was tested in mice, and this strategy induced high titers of neutralizing antibodies and a robust TCD4+ response. Additionally, after nasal challenge with H5N2 virus, animals were found to be significantly protected [110]. The same polyanhydride NP system was tested to generate protection against swine influenza A virus (SwIAV) in pigs. After two immunization steps with the NP vaccine loaded with inactivated H1N2, pigs challenged with SwIAV showed less-severe clinical and pathological symptoms compared with animals immunized with free antigen. In addition, the levels of CD8+IFNγ+, CD4+IFNγ+ and CD3+ IFN-γ+ (NK) cells were higher in the vaccinated group [111]. The application of an NP system to influenza was also tested using avian H7N9 antigens as VLPs. In this model, a VLP expressing the antigen combination of HA, NA and M1 from the strain A/Taiwan/S02076/2013 (H7N9) induced a strong HI antibody response after primary and booster vaccination. This was accompanied by T cell immunity, including the production of IL-4 and TNF by CD4+ and CD8+ T cells, respectively [112]. The benefits of mucosal immunization with NPs were clearly observed in chickens immunized via the aerosol route. In that study, PLGA loaded with formalin-inactivated H9N2 virus and CpG generated a significantly stronger antibody response, as indicated by the HI titer, than non-encapsulated forms. The authors attributed this result to an increase in the length of time the immunogen remains at the respiratory mucosal surface, thereby facilitating interactions with APCs [113]. In addition to mucosal protection, another challenge for influenza vaccine development is the induction of cross-protective antibodies that target different pathways involved in influenza virus infection. This goal was achieved by linking two different conserved influenza antigens (the ectodomain of matrix protein 2 (M2e) and helix C) in a self-assembling protein nanoparticle (SAPN), which maintains the native tetrameric structure of the antigens. The SAPN/M2e/helix C induced neutralizing antibodies and cross-protection in avian and mouse models [114].
Obstacles and future perspectives
NP vaccines are systems that have great potential in the development and optimization of viral vaccines for public health and prophylaxis. However, the protective efficacy of nanostructures against viruses which use different routes of infection needs to be improved and better characterized. We still do not know if an oral or nasal NP vaccine will provide long-term mucosal immunity against a specific virus, or if protection will result when administered at different ages or via different routes. The models that have already been characterized should be tested with viruses that have different mechanisms of infection and biology. The effect of viral NP vaccines on cell populations such as central, effector, and tissue-resident memory T cells, regulatory T cells, and cytokines involved in adaptive immunity (IL-7, IL-21, IL-15) have not been adequately investigated. We still do not know if an NP vaccine can cause some level of long-term toxicity. More studies are needed to investigate possible local or systemic damage. To date, there are no specific clinical trials for viral NP vaccines listed in ClinicalTrial.gov. Using the term NP, 274 clinical trials (including completed, recruiting, withdrawn and unknown) were found that were related to cancer and chronic diseases. This lack of clinical trials reflects the necessity for optimization of viral NP vaccines. Several unknown aspects need to be clarified before viral NP vaccines reach the industrial-scale production stage and positively impact in public health.
References
Lien G, Heymann DL (2013) The problems with polio: toward eradication. Infect Dis Ther 2:167–174
Chappuis F, Farinelli T, Deckx H et al (2017) Immunogenicity and estimation of antibody persistence following vaccination with an inactivated virosomal hepatitis A vaccine in adults: a 20-year follow-up study. Vaccine 35:1448–1454. https://doi.org/10.1016/j.vaccine.2017.01.031
Paz-Zulueta M, Álvarez-Paredes L, Rodríguez Díaz JC et al (2018) Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer. https://doi.org/10.1186/s12885-018-4033-2
Pezzotti P, Bellino S, Prestinaci F et al (2018) The impact of immunization programs on 10 vaccine preventable diseases in Italy: 1900–2015. Vaccine. https://doi.org/10.1016/j.vaccine.2018.01.065
Nuismer SL, Althouse BM, May R et al (2016) Eradicating infectious disease using weakly transmissible vaccines. Proc R Soc B Biol Sci 283:20161903. https://doi.org/10.1098/rspb.2016.1903
Carole Tevi-Benissan M, Moturi E, Anya BPM et al (2016) Contribution of polio eradication initiative to effective new vaccine introduction in Africa, 2010–2015. Vaccine 34:5193–5198. https://doi.org/10.1016/j.vaccine.2016.05.063
Shaalan M, Saleh M, El-Mahdy M, El-Matbouli M (2016) Recent progress in applications of nanoparticles in fish medicine: a review. Nanomed Nanotechnol Biol Med 12:701–710. https://doi.org/10.1016/j.nano.2015.11.005
Nandedkar TD (2009) Nanovaccine : recent developments in vaccination. Proteins 34:995–1003. https://doi.org/10.1007/s12038-009-0114-3
Peek LJ, Middaugh CR, Berkland C (2008) Nanotechnology in vaccine delivery. Adv Drug Deliv Rev 60:915–928. https://doi.org/10.1016/j.addr.2007.05.017
Zhao L, Seth A, Wibowo N et al (2014) Nanoparticle vaccines. Vaccine 32:327–337. https://doi.org/10.1016/j.vaccine.2013.11.069
Mahapatro A, Singh DK (2011) Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol 9:55. https://doi.org/10.1186/1477-3155-9-55
Barani H, Montazer M (2008) A review on applications of liposomes in textile processing. J Liposome Res 18:249–262. https://doi.org/10.1080/08982100802354665
Chiu CC, Moore PB, Shinoda W, Nielsen SO (2009) Size-dependent hydrophobic to hydrophilic transition for nanoparticles: a molecular dynamics study. J Chem Phys 131:1–9. https://doi.org/10.1063/1.3276915
Crozat K, Guiton R, Contreras V et al (2010) The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med 207:1283–1292. https://doi.org/10.1084/jem.20100223
Zolnik BS, González-Fernández Á́, Sadrieh N, Dobrovolskaia MA (2010) Minireview: nanoparticles and the immune system. Endocrinology 151:458–465. https://doi.org/10.1210/en.2009-1082
Arama C, Giusti P, Boström S et al (2011) Interethnic differences in antigen-presenting cell activation and TLR responses in malian children during Plasmodium falciparum malaria. PLoS One. https://doi.org/10.1371/journal.pone.0018319
Barry AE, Arnott A (2014) Strategies for designing and monitoring malaria vaccines targeting diverse antigens. Front Immunol 5:359. https://doi.org/10.3389/fimmu.2014.00359
Bolhassani A, Javanzad S, Saleh T et al (2014) Polymeric nanoparticles potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum Vaccines Immunother 10:321–323. https://doi.org/10.4161/hv.26796
Mocan T (2014) Nanoparticles-based cancer vaccines. Biotechnol Mol Biol Nanomed 2:13–14
Wadhwa S, Jain A, Woodward JG, Mumper RJ (2013) Lipid nanocapsule as vaccine carriers for his-tagged proteins: evaluation of antigen-specific immune responses to HIV I His-Gag p41 and systemic inflammatory responses. Eur J Pharm Biopharm 80:315–322. https://doi.org/10.1016/j.ejpb.2011.10.016.Lipid
Zaric M, Lyubomska O, Touzelet O et al (2013) Skin dendritic cell targeting via microneedle arrays laden with co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano 7:2042–2055
Christensen D, Korsholm KS, Andersen P, Agger EM (2011) Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines 10:513–521
Silva AL, Soema PC, Slütter B et al (2016) PLGA particulate delivery systems for subunit vaccines: linking particle properties to immunogenicity. Hum Vaccines Immunother 12:1056–1069
Moon JJ, Suh H, Bershteyn A et al (2011) Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater 10:243–251. https://doi.org/10.1038/nmat2960.Interbilayer-Crosslinked
Cordeiro AS, Alonso MJ, de la Fuente M (2015) Nanoengineering of vaccines using natural polysaccharides. Biotechnol Adv 33:1279–1293
Trovato M, De Berardinis P (2015) Novel antigen delivery systems. World J Virol 4:156–168. https://doi.org/10.5501/wjv.v4.i3.156
Sanders MT, Brown LE, Deliyannis G, Pearse MJ (2005) ISCOMTM-based vaccines: the second decade. Immunol Cell Biol 83:119–128
Kushnir N, Streatfield SJ, Yusibov V (2012) Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine 31:58–83. https://doi.org/10.1016/j.vaccine.2012.10.083
Jeong H, Seong BL (2017) Exploiting virus-like particles as innovative vaccines against emerging viral infections. J Microbiol 55:220–230. https://doi.org/10.1007/s12275-017-7058-3
Zhao Q, Li S, Yu H et al (2013) Virus-like particle-based human vaccines: quality assessment based on structural and functional properties. Trends Biotechnol 31:654–663. https://doi.org/10.1016/j.tibtech.2013.09.002
Wang Y, Wang Y, Kang N et al (2016) Construction and immunological evaluation of CpG-Au @ HBc virus-like nanoparticles as a potential vaccine. Nanoscale Res Lett. https://doi.org/10.1186/s11671-016-1554-y
Mach H, Volkin DB, Troutman RD et al (2006) Disassembly and reassembly of yeast-derived recombinant human papillomavirus virus-like particles (HPV VLPs). J Pharm Sci 95:2195–2206. https://doi.org/10.1002/jps.20696
Binjawadagi B, Lakshmanappa YS, Longchao Z et al (2016) Development of a porcine reproductive and respiratory syndrome virus-like-particle-based vaccine and evaluation of its immunogenicity in pigs. Arch Virol 161:1579–1589. https://doi.org/10.1007/s00705-016-2812-0
Dhakal S, Hiremath J, Bondra K et al (2017) Biodegradable nanoparticle delivery of inactivated swine influenza virus vaccine provides heterologous cell-mediated immune response in pigs. J Control Release 247:194–205. https://doi.org/10.1016/j.jconrel.2016.12.039
Amirnasr M, Fallah tafti T, Sankian M et al (2016) Immunization against HTLV-I with chitosan and tri-methylchitosan nanoparticles loaded with recombinant env23 and env13 antigens of envelope protein gp46. Microb Pathog 97:38–44. https://doi.org/10.1016/j.micpath.2016.05.012
Kim SY, Noh YW, Kang TH et al (2017) Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials 130:56–66. https://doi.org/10.1016/j.biomaterials.2017.03.034
Gutjahr A, Phelip C, Coolen A-L et al (2016) Biodegradable polymeric nanoparticles-based vaccine adjuvants for lymph nodes targeting. Vaccines 4:34. https://doi.org/10.3390/vaccines4040034
Xiang J, Xu L, Gong H et al (2015) Antigen-loaded upconversion nanoparticles for dendritic cell stimulation, tracking, and vaccination in dendritic cell-based immunotherapy. ACS Nano 9:6401–6411. https://doi.org/10.1021/acsnano.5b02014
Reddy ST, Rehor A, Schmoekel HG et al (2006) In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J Control Release 112:26–34. https://doi.org/10.1016/j.jconrel.2006.01.006
Reddy ST, Van Der Vlies J, Simeoni E et al (2007) Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 25:1159–1164. https://doi.org/10.1038/nbt1332
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol 11:373–384. https://doi.org/10.1038/ni.1863
Suresh R, Mosser DM (2013) Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv Physiol Educ 37:284–291. https://doi.org/10.1152/advan.00058.2013
Chiang C, Gack MU (2017) Post-translational control of intracellular pathogen sensing pathways. Trends Immunol 38:39–52. https://doi.org/10.1016/j.it.2016.10.008
Kato H, Oh S-W, Fujita T (2017) RIG-I-like receptors and type I interferonopathies. J Interf Cytokine Res 37:207–213. https://doi.org/10.1089/jir.2016.0095
Lee Y, Lee Y, Kim K et al (2013) Induction of potent antigen-specific cytotoxic T cell response by PLGA-nanoparticles containing antigen and TLR agonist. Immune Netw 13:30–33
Li AV, Moon JJ, Abraham W et al (2013) Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination adrienne. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3006516.Generation
Mohsen MO, Gomes AC, Cabral-Miranda G et al (2017) Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. J Control Release 251:92–100. https://doi.org/10.1016/j.jconrel.2017.02.031
Lv S, Wang J, Dou S et al (2014) Nanoparticles encapsulating hepatitis B virus cytosine–phosphate–guanosine induce therapeutic immunity against HBV infection. Hepatology 59:385–394. https://doi.org/10.1002/hep.26654
Ebrahimian M, Hashemi M, Maleki M et al (2017) Co-delivery of dual toll-like receptor agonists and antigen in poly(lactic-co-glycolic) acid/polyethylenimine cationic hybrid nanoparticles promote efficient in vivo immune responses. Front Immunol. https://doi.org/10.3389/fimmu.2017.01077
Leleux JA, Pradhan P, Roy K (2017) Biophysical attributes of CpG presentation control TLR9 signaling to differentially polarize systemic immune responses. Cell Rep 18:700–710. https://doi.org/10.1016/j.celrep.2016.12.073
Mukai Y, Yoshinaga T, Yoshikawa M et al (2011) Induction of endoplasmic reticulum-endosome fusion for antigen cross-presentation induced by poly (-glutamic acid) nanoparticles. J Immunol 187:6249–6255. https://doi.org/10.4049/jimmunol.1001093
Molino NM, Anderson AKL, Nelson EL, Wang SW (2013) Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano 7:9743–9752. https://doi.org/10.1021/nn403085w
Turner LH, Kinder JM, Wilburn A et al (2017) Preconceptual Zika virus asymptomatic infection protects against secondary prenatal infection. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1006684
Pileggi C, Papadopoli R, Bianco A, Pavia M (2017) Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine 35:6302–6307. https://doi.org/10.1016/j.vaccine.2017.09.076
Ng S, Saborio S, Kuan G et al (2017) Association between haemagglutination inhibiting antibodies and protection against clade 6B viruses in 2013 and 2015. Vaccine 35:6202–6207. https://doi.org/10.1016/j.vaccine.2017.09.036
Reikie BA, Naidoo S, Ruck CE et al (2013) Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. Clin Vaccine Immunol 20:33–38. https://doi.org/10.1128/CVI.00557-12
Bachmann MF, Jennings GT (2010) Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10:787–796
Avalos AM, Ploegh HL (2014) Early BCR events and antigen capture, processing, and loading on MHC class II on B cells. Front, Immunol, p 5
Moon JJ, Suh H, Li AV et al (2012) Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci 109:1080–1085. https://doi.org/10.1073/pnas.1112648109
Agarwal R, Singh V, Jurney P et al (2013) Mammalian cells preferentially internalize hydrogel nanodiscs over nanorods and use shape-specific uptake mechanisms. Proc Natl Acad Sci 110:17247–17252. https://doi.org/10.1073/pnas.1305000110
Zhao F, Zhao Y, Liu Y et al (2011) Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 7:1322–1337
Rosette C, Karin M (1995) Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-κB. J Cell Biol 128:1111–1119. https://doi.org/10.1083/jcb.128.6.1111
Jans J, elMoussaoui H, de Groot R et al (2016) Actin- and clathrin-dependent mechanisms regulate interferon gamma release after stimulation of human immune cells with respiratory syncytial virus. Virol J 13:52. https://doi.org/10.1186/s12985-016-0506-6
Piccini LE, Castilla V, Damonte EB (2015) Dengue-3 virus entry into vero cells: role of clathrin-mediated endocytosis in the outcome of infection. PLoS One 10:1–17. https://doi.org/10.1371/journal.pone.0140824
Cruz-Oliveira C, Freire JM, Conceição TM et al (2015) Receptors and routes of dengue virus entry into the host cells. FEMS Microbiol Rev 39:155–170. https://doi.org/10.1093/femsre/fuu004
Benedicto I, Gondar V, Molina-Jiménez F et al (2015) Clathrin mediates infectious hepatitis C virus particle egress. J Virol 89:4180–4190. https://doi.org/10.1128/JVI.03620-14
Wu B, Geng S, Bi Y et al (2015) Herpes simplex virus 1 suppresses the function of lung dendritic cells via caveolin-1. Clin Vaccine Immunol 22:883–895. https://doi.org/10.1128/cvi.00170-15
Hu Y, Ehrich M, Fuhrman K, Zhang C (2014) In vitro performance of lipid-PLGA hybrid nanoparticles as an antigen delivery system: lipid composition matters. Nanoscale Res Lett 9:1–10
Weyland M, Griveau A, Bejaud J et al (2013) Lipid nanocapsule functionalization by lipopeptides derived from human papillomavirus type-16 capsid for nucleic acid delivery into cancer cells. Int J Pharm 454:756–764. https://doi.org/10.1016/j.ijpharm.2013.06.013
Walczak AP, Hendriksen PJM, Woutersen RA et al (2015) Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J Nanopart Res 17:231. https://doi.org/10.1007/s11051-015-3029-y
Bernkop-Schnürch A, Greimel A (2005) Thiomers: the next generation of mucoadhesive polymers. Am J Drug Deliv 3:141–154. https://doi.org/10.2165/00137696-200503030-00001
Salazar-González JA, González-Ortega O, Rosales-Mendoza S (2015) Gold nanoparticles and vaccine development. Expert Rev Vaccines 14:1197–1211. https://doi.org/10.1586/14760584.2015.1064772
Ebrahimian M, Hashemi M, Maleki M et al (2016) Induction of a balanced Th1/Th2 immune responses by co-delivery of PLGA/ovalbumin nanospheres and CpG ODNs/PEI-SWCNT nanoparticles as TLR9 agonist in BALB/c mice. Int J Pharm 515:708–720. https://doi.org/10.1016/j.ijpharm.2016.10.065
Mahony D, Cavallaro AS, Mody KT et al (2014) In vivo delivery of bovine viral diahorrea virus, E2 protein using hollow mesoporous silica nanoparticles. Nanoscale 6:6617. https://doi.org/10.1039/c4nr01202j
Toda T, Yoshino S (2016) Enhancement of ovalbumin-specific Th1, Th2, and Th17 immune responses by amorphous silica nanoparticles. Int J Immunopathol Pharmacol 29:408–420. https://doi.org/10.1177/0394632016656192
Liu T, Liu H, Fu C et al (2013) Silica nanorattle with enhanced protein loading: a potential vaccine adjuvant. J Colloid Interface Sci 400:168–174. https://doi.org/10.1016/j.jcis.2013.03.005
Seong S-Y, Matzinger P (2004) Opinion: hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 4:469–478. https://doi.org/10.1038/nri1372
Moyano DF, Goldsmith M, Solfiell DJ et al (2012) Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc 134:1–7
Metz SW, Tian S, Hoekstra G et al (2016) Precisely molded nanoparticle displaying DENV-E proteins induces robust serotype-specific neutralizing antibody responses. PLoS Negl Trop Dis 10:1–17. https://doi.org/10.1371/journal.pntd.0005071
Hunsawong T, Sunintaboon P, Warit S et al (2015) A novel dengue virus serotype-2 nanovaccine induces robust humoral and cell-mediated immunity in mice. Vaccine 33:1702–1710. https://doi.org/10.1016/j.vaccine.2015.02.016
Hunsawong T, Sunintaboon P, Warit S et al (2015) Immunogenic properties of a BCG adjuvanted chitosan nanoparticle-based dengue vaccine in human dendritic cells. PLoS Negl Trop Dis 9:e0003958. https://doi.org/10.1371/journal.pntd.0003958
Nantachit N, Sunintaboon P, Ubol S (2016) Responses of primary human nasal epithelial cells to EDIII-DENV stimulation: the first step to intranasal dengue vaccination. Virol J. https://doi.org/10.1186/s12985-016-0598-z
World Health Organization (WHO) (2017) Hepatitis B vaccines: WHO position paper—July 2017. Wkly Epidemiol Rec 27:369–392. https://doi.org/10.1186/1750-9378-2-15.Voir
El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142(6):1264–1273. https://doi.org/10.1053/j.gastro.2011.12.061
Shaw CA, Tomljenovic L (2013) Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res 56:304–316. https://doi.org/10.1007/s12026-013-8403-1
Tomljenovic L, Shaw CA (2012) Mechanisms of aluminum adjuvant toxicity and autoimmunity in pediatric populations. Lupus 21:223–230
HogenEsch H (2012) Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. https://doi.org/10.3389/fimmu.2012.00406
Bharali DJ, Pradhan V, Elkin G et al (2008) Novel nanoparticles for the delivery of recombinant hepatitis B vaccine. Nanomed Nanotechnol Biol Med 4:311–317. https://doi.org/10.1016/j.nano.2008.05.006
Chiara F, Bartolucci GB, Cattai M et al (2013) Hepatitis B vaccination of adolescents: significance of non-protective antibodies. Vaccine 32:62–68. https://doi.org/10.1016/j.vaccine.2013.10.074
Sahu KK, Pandey RS (2016) Immunological evaluation of colonic delivered Hepatitis B surface antigen loaded TLR-4 agonist modified solid fat nanoparticles. Int Immunopharmacol 39:343–352. https://doi.org/10.1016/j.intimp.2016.08.007
Dinda AK, Bhat M, Srivastava S et al (2016) Novel nanocarrier for oral hepatitis B vaccine. Vaccine 34:3076–3081. https://doi.org/10.1016/j.vaccine.2016.04.084
Jesus S, Soares E, Borchard G, Borges O (2017) Poly-ϵ-caprolactone/chitosan nanoparticles provide strong adjuvant effect for hepatitis B antigen. Nanomedicine. https://doi.org/10.2217/nnm-2017-0138
Pietrzak-Nguyen A, Piradashvili K, Fichter M et al (2016) MPLA-coated hepatitis B virus surface antigen (HBsAg) nanocapsules induce vigorous T cell responses in cord blood derived human T cells. Nanomed Nanotechnol Biol Med 12:2383–2394. https://doi.org/10.1016/j.nano.2016.07.010
Ahmed Y, Tian M, Gao Y (2017) Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies. AIDS Res, Ther, p 14
Julg B, Tartaglia LJ, Keele BF et al (2017) Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aal1321
Korber B, Hraber P, Wagh K, Hahn BH (2017) Polyvalent vaccine approaches to combat HIV-1 diversity. Immunol Rev 275:230–244
Caskey M, Klein F, Nussenzweig MC (2016) Broadly neutralizing antibodies for HIV-1 prevention or immunotherapy. N Engl J Med 375:2019–2021. https://doi.org/10.1056/NEJMp1613362
Sanders RW, Van Gils MJ, Derking R et al (2015) HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. https://doi.org/10.1126/science.aac4223
Sliepen K, Ozorowski G, Burger JA et al (2015) Presenting native-like HIV -1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology. https://doi.org/10.1186/s12977-015-0210-4
He L, de Val N, Morris CD et al (2016) Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat Commun 7:12041. https://doi.org/10.1038/ncomms12041
Brewer MG, DiPiazza A, Acklin J et al (2017) Nanoparticles decorated with viral antigens are more immunogenic at low surface density. Vaccine 35:774–781. https://doi.org/10.1016/j.vaccine.2016.12.049
Bale S, Goebrecht G, Stano A et al (2017) Covalent linkage of HIV-1 trimers to synthetic liposomes elicits improved B cell and antibody responses. J Virol 91:e00443-17. https://doi.org/10.1128/JVI.00443-17
Rostami H, Ebtekar M, Shafiee MAMHY, Mahdavi M (2017) Co-utilization of a TLR5 agonist and nano-formulation of HIV-1 vaccine candidate leads to increased vaccine immunogenicity and decreased immunogenic dose: a preliminary studypt. Immunol Lett 187:19–26. https://doi.org/10.1016/j.imlet.2017.05.002
Pejawar-gaddy S, Kovacs JM, Barouch DH et al (2015) Design of lipid nanocapsule delivery vehicles for multivalent display of recombinant Env trimers in HIV vaccination. Bioconjug Chem 25:1470–1478
Aikins ME, Bazzill J, Moon J (2017) Vaccine nanoparticles for protection against HIV infection. Future Med 12:673–682
Muszkat M, Greenbaum E, Ben-Yehuda A et al (2003) Local and systemic immune response in nursing-home elderly following intranasal or intramuscular immunization with inactivated influenza vaccine. Vaccine 21:1180–1186. https://doi.org/10.1016/S0264-410X(02)00481-4
Su F, Patel GB, Hu S, Chen W (2016) Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum Vaccines Immunother 12:1070–1079
Rioux G, Mathieu C, Russell A et al (2014) PapMV nanoparticles improve mucosal immune responses to the trivalent inactivated flu vaccine. J Nanobiotechnol. https://doi.org/10.1186/1477-3155-12-19
Dehghan S, Tafaghodi M, Bolourieh T et al (2014) Rabbit nasal immunization against influenza by dry-powder form of chitosan nanospheres encapsulated with influenza whole virus and adjuvants. Int J Pharm 475:1–8. https://doi.org/10.1016/j.ijpharm.2014.08.032
Ross KA, Wu W, Huntimer L et al (2015) Hemagglutinin-based polyanhydride nanovaccines against H5N1 influenza elicit protective virus neutralizing titers and cell-mediated immunity. Int J Nanomed 30(10):229–243. https://doi.org/10.2147/IJN.S72264
Dhakal S, Goodman J, Bondra K et al (2017) Polyanhydride nanovaccine against swine influenza virus in pigs. Vaccine. https://doi.org/10.1016/j.vaccine.2017.01.019
Hu CJ, Chien C, Liu M et al (2017) Multi-antigen avian influenza a (H7N9) virus-like particles: particulate characterizations and immunogenicity evaluation in murine and avian models. BMC Biotechnol. https://doi.org/10.1186/s12896-016-0321-6
Singh SM, Alkie TN, Abdelaziz KT et al (2016) Characterization of immune responses to an inactivated avian influenza virus vaccine adjuvanted with nanoparticles containing CpG ODN. Viral Immunol 29:269–275. https://doi.org/10.1089/vim.2015.0144
Karch CP, Li J, Kulangara C et al (2017) Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge. Nanomed Nanotechnol Biol Med 13:241–251. https://doi.org/10.1016/j.nano.2016.08.030
Somiya M, Yoshimoto N, Iijima M et al (2012) Targeting of polyplex to human hepatic cells by bio-nanocapsules, hepatitis B virus surface antigen L protein particles. Bioorg Med Chem 20:3873–3879. https://doi.org/10.1016/j.bmc.2012.04.031
Moon JJ, Suh H, Bershteyn A et al (2011) Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater 10:243–251. https://doi.org/10.1038/nmat2960.Interbilayer-Crosslinked
Jesus S, Soares E, Costa J et al (2016) Immune response elicited by an intranasally delivered HBsAg low-dose adsorbed to poly-ε-caprolactone based nanoparticles. Int J Pharm 504:59–69. https://doi.org/10.1016/j.ijpharm.2016.03.013
Zhao M, Li M, Zhang Z et al (2015) Induction of HIV-1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv 1:1–12
Lv S, Wang J, Dou S et al (2013) Nanoparticles encapsulating HBV-CpG induce therapeutic immunity against hepatitis B virus infection. Hepatology. https://doi.org/10.1002/hep.26654
Metz SW, Tian S, Hoekstra G et al (2016) Precisely molded nanoparticle displaying DENV-E proteins induces robust serotype-specific neutralizing antibody responses. PLoS Negl Trop Dis 10:1–17. https://doi.org/10.1371/journal.pntd.0005071
Ebensen T, Debarry J, Pedersen GK et al (2017) Mucosal administration of cycle-di-nucleotide-adjuvanted virosomes efficiently induces protection against influenza H5N1 in mice. Front Immunol. https://doi.org/10.3389/fimmu.2017.01223
Turmagambetova AS, Alexyuk PG, Bogoyavlenskiy AP et al (2017) Adjuvant activity of saponins from Kazakhstani plants on the immune responses to subunit influenza vaccine. Arch Virol 162:3817–3826. https://doi.org/10.1007/s00705-017-3560-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Chan-Shing Lin.
Rights and permissions
About this article
Cite this article
Sulczewski, F.B., Liszbinski, R.B., Romão, P.R.T. et al. Nanoparticle vaccines against viral infections. Arch Virol 163, 2313–2325 (2018). https://doi.org/10.1007/s00705-018-3856-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3856-0