Abstract
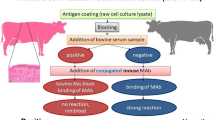
Peste des petits ruminants (PPR) is a contagious and economically important disease affecting production of small ruminants (i.e., sheep and goats). Taking into consideration the lessons learnt from the Global Rinderpest Eradication Programme (GREP), PPR is now targeted by the international veterinary community as the next animal disease to be eradicated. To support the African continental programme for the control of PPR, the Pan African Veterinary Vaccine Centre of the African Union (AU-PANVAC) is developing diagnostics tools. Here, we describe the development of a blocking enzyme-linked immunosorbent assay (bELISA) that allows testing of a large number of samples for specific detection of antibodies directed against PPR virus in sheep and goat sera. The PPR bELISA uses an anti-haemagglutinin (H) monoclonal antibody (MAb) as a competitor antibody, and tests results are interpreted using the percentage of inhibition (PI) of MAb binding generated by the serum sample. PI values below or equal to 18% (PI ≤ 18%) are negative, PI values greater than or equal to 25% (PI ≥ 25%) are positive, and PI values greater than 18% and below 25% are doubtful. The diagnostic specificity (DSp) and diagnostic sensitivity (DSe) were found to be 100% and 93.74%, respectively. The H-based PPR-bELISA showed good correlation with the virus neutralization test (VNT), the gold standard test, with a kappa value of 0.947. The H-based PPR-bELISA is more specific than the commercial kit ID Screen® PPR Competition (N-based PPR-cELISA) from IDvet (France), but the commercial kit is slightly more sensitive than the H-based PPR-bELISA. The validation process also indicated good repeatability and reproducibility of the H-based PPR-bELISA, making this new test a suitable tool for the surveillance and sero-monitoring of the vaccination campaign.






Similar content being viewed by others
Abbreviations
- AU-IBAR:
-
African Union Inter-African Bureau for Animal Resources
- AU-PANVAC:
-
African Union Pan-African Veterinary Vaccine Centre
- bELISA:
-
Blocking enzyme-linked immunosorbent assay
- cELISA:
-
Competitive enzyme-linked immunosorbent assay
- dpv:
-
Days post-vaccination
- ELISA:
-
Enzyme-linked immunosorbent assay
- FAO:
-
Food and Agriculture Organisation of the United Nations
- GREP:
-
Global Rinderpest Eradication Programme
- OIE:
-
World Organization for Animal Health
- PPR:
-
Peste des petits ruminants
- PPRV:
-
Peste des petits ruminants virus
- VNT:
-
Virus neutralisation test
References
Kitching RP (1988) The economic significance and control of small ruminant viruses in North Africa and West Asia. In: Thompson FS (ed) Increasing small ruminant productivity in semi-arid areas. Kluwer Academic Publishers, Dordrecht, pp 225–236
Libeau G, Diallo A, Parida S (2014) Evolutionary genetics underlying the spread of peste des petits ruminants virus. Anim Front 4:14–20
Gibbs EPJ, Taylor WP, Lawman MJP, Bryant J (1979) Classification of peste des petits ruminants virus as the fourth member of the Genus Morbillivirus. Intervirology 11:268–274
Diallo A (1990) Morbillivirus group: genome organization and proteins. Vet Microbiol 23:155–163
Barrett T, Visser IKG, Mamacv L, Goatley L, Van Bressem MF, Osterhaus ADME (1993) Dolphin and porpoise morbilliviruses are genetically distinct from phocine distemper virus. Virology 193:1010–1012
Banyard AC, Rima BK, Barret T (2006) The morbilliviruses. In: Barrett T, Pastoret PP, Taylor WP (eds) Rinderpest and peste des petits ruminants. Virus plagues of large and small ruminants, Elsevier, pp 31–67
Gargadennec L, Lalanne A (1942) La peste des petits ruminants. Bull Serv Zoo AOF 5:16–21
Diallo A (2013) Global distribution and evolution of PPR: understanding the lineage evolution, the gaps, the challenges and research priorities. IAEA/FAO/OIE Workshop on PPR in the SADC Region (Dar es Salaam, 10–12 June 2013). http://www.rr-africa.oie.int/docspdf/en/2013/PPR/DIALLO.pdf. Accessed 10 Feb 2015
Lefèvre PC, Diallo A, Schenke F, Hussein S, Staak G (1991) Serological evidence of peste des petits ruminants in Jordan. Vet Rec 128:110
Amjad H, Ul-Islam Q, Forsyth M, Barrett T, Rossiter P (1996) Peste des petits ruminants in Pakistan. Vet Rec 139:118–119
Shaila MS, Shamaki D, Forsyth MA, Diallo A, Goatley L, Kitching RP, Barrett T (1996) Geographic distribution and epidemiology of peste des petits ruminants virus. Virus Res 43:149–153
Ozkul A, Akca Y, Alkan F, Barrett T, Karaoglu T, Daglap SB, Anderson J, Yesilbag K, Cokcaliskan C, Genacy A, Burgu I (2002) Prevalence, distribution and host range of peste des petits ruminants virus, Turkey. Emerg Infect Dis 8:708–712
Kwiatek O, Minet C, Grillet C, Hurard C, Carlsson E, Karimov B, Albina E, Diallo A, Libeau G (2007) Peste des petits ruminants (PPR) outbreak in Tajikistan. J Comp Pathol 136:111–119
Al-Dubaib MA (2008) Prevalence of peste des petits ruminants virus infection in sheep and goat farms at the central region of Saudi Arabia. Res J Vet Sci 1:67–70
Al-Dubaib MA (2009) Peste des petits ruminants morbillivirus infection in lambs and young goats at Qassim region, Saudi Arabia. Trop Anim Health Prod 41:217–220
Albayrak H, Alkan F (2009) PPR virus infection of sheep in black sea region of Turkey: epidemiology and diagnosis by RT-PCR and virus isolation. Vet Res Commun 33:241–249
Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G (2010) Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol 91:2885–2897
Libeau G, Diallo A, Colas F, Guerre L (1994) Rapid differential diagnosis of rinderpest and peste des petits ruminants using an immunocapture ELISA. Vet Rec 134:300–304
Forsyth MA, Barrett T (1995) Evaluation of polymerase chain reaction for the detection and characterisation of rinderpest and peste des petits ruminants viruses for epidemiological studies. Virus Res 39:151–163
Couacy-Hymann E, Roger F, Hurard C, Guillou JP, Libeau G, Diallo A (2002) Rapid and sensitive detection of peste des petits ruminants virus by a polymerase chain reaction assay. J Virol Methods 100:17–25
Kwiatek O, Keita D, Gil P, Fernandez-Pinero J, Jimenez Clavero MA, Albina E, Libeau G (2010) Quantitative one-step real-time RT-PCR for the fast detection of the four genotypes of PPRV. J Virol Methods 165:168–177
Kwiatek O, Minet C, Grillet C, Hurard C, Carlsson E, Karimov B, Albina E, Diallo A, Libeau G (2007) Peste des petits ruminants (PPR) outbreak in Tajikistan. J Comp Pathol 136:111–119
Adombi CM, Lelenta M, Lamien CE, Shamaki D, Koffi YM, Traoré A, Silber R, Couacy-Hymann E, Bodjo SC, Djaman JA, Luckins AG, Diallo A (2011) Monkey CV1 Cell line expressing the sheep-1 goat SLAM protein: a highly sensitive cell line for the isolation of peste des petits ruminants virus from pathological specimens. J Virol Methods 173:306–313
Anderson J, McKay JA (1994) The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implications to rinderpest control programmes. Epidemiol Infect 112:225–231
Libeau G, Prehaud C, Lancelot R, Colas F, Guerre L, Bishop DH, Diallo A (1995) Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleoprotein. Res Vet Sci 58:50–55
Choi KS, Nah JJ, Ko YJ, Kang SY, Jo NI (2005) Rapid competitive enzyme-linked immunosorbent assay for detection of antibodies to peste des petits ruminants virus. Clin Diagn Lab Immunol 12:542–547
Diallo A, Taylor WP, Lefèvre PC, Provost A (1989) Atténuation d’une souche du virus de la PPR. Candidat pour un vaccin homologue vivant. Rev Elev Méd vét Pays Trop 42:311–319
Diallo A (2003) Control of peste des petits ruminants: classical and new generation vaccines. Dev Biol 114:113–119
The World Organization for Animal Health-OIE (2014). Resolutions adopted during the by the Delegates of the OIE during the 82nd General Assembly 25–30 May
Food Agriculture Organisation of United Nation (FAO), World Organization for Animal Health-OIE (2015) In: International conference for the control and eradication of PPR (Abidjan, Cote d’Ivoire, 31 March–03 April 2015). The global strategy for the control and eradication of PPR. http://www.oie.int/fr/PPR2015/doc/PPR-Global-Strategy-avecAnnexes_2015-03-28.pdf. Accessed 10 Apr 2016
FAO (2012) Lessons learned from the eradication of rinderpest for controlling other transboundary animal diseases. In: Proceedings of the GREP symposium and high-level meeting (Rome, Italy, 12–15 October 2010). http://www.fao.org/3/a-i3042e.pdf. Accessed 22 Feb 2016
Nwankpa N, Bodjo C, Tounkara K, Domenech J (2015) Quality control of PPR vaccine in Africa: the role of AU-PANVAC. OIE Bull. 2:64–71
Anderson J, McKay JA, Butcher RN (1991). The use of monoclonal antibodies in competitive ELISA for the detection of antibodies to rinderpest and peste des petits ruminants viruses. In: Seromonitoring of Rinderpest throughout Africa: Phase One. Proceedings of the final research coordination meeting of the IAEA rinderpest control projects, Cote d’Ivoire 19–23 November 1990. IAEA-TECDOC-623 Vienna International Atomic Energy Agency
Rossiter PB, Jessett DM, Taylor WP (1985) Microneutralisation systems for use with different strains of peste des petits ruminants virus and rinderpest virus. Trop Anim Health Prod 17:75–81
Bodjo SC, Lelenta M, Couacy-Hymann E, Kwiatek O, Albina E, Gargani D, Libeau G, Diallo A (2008) Mapping the peste des petits ruminants virus nucleoprotein: identification of two domains involved in protein self-association. Virus Res 131:23–32
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Cohen JA (1960) Coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
Ferrin NH, Fang Y, Johnson CR, Murtaugh MP, Polson DD, Torremorell M, Gramer ML, Nelson EA (2004) Validation of a blocking enzyme-linked immunosorbent assay for detection of antibodies against porcine reproductive and respiratory syndrome virus. Clin Diagn Lab Immunol 11:503–514
Sorensen KJ, Botner A, Madsen ES, Strandbygaard B, Nielsen J (1997) Evaluation of a blocking ELISA for screening of antibodies against porcine reproductive and respiratory syndrome (PRRS) virus. Vet Microbiol 56:1–8
Houben S, Callebaut P, Pensaert MB (1995) Comparative study of a blocking enzyme-linked immunosorbent assay and the immunoperoxidase monolayer assay for the detection of antibodies to the porcine reproductive and respiratory syndrome virus in pigs. J Virol Methods 51:125–128
Rima Bert K (1983) The proteins of morbilliviruses. J Gen Virol 64:1205–1219
Ogura H, Sato H, Kamiya S, Nakamura S (1991) Glycosylation of measles virus haemmaglutinin protein in infected cells. J Gen Virol 72:2679–2684
Langedijk JPM, Daus FJ, Van Oirschot JT (1997) Sequence and structure alignment of Paramyxoviridae attachment proteins and discovery of enzymatic activity for a morbillivirus hemagglutinin. J Virol 71:6155–6167
Corey EA, Iorio RM (2007) Mutations in the stalk of the measles virus hemagglutinin protein decrease fusion but do not interfere with virus-specific interaction with the homologous fusion protein. J Virol 81:9900–9910
Grubman MJ, Mebus C, Dale B, Yamanaka M, Yilma T (1988) Analysis of the polypeptides synthesized in rinderpest virus-lnfected cells. Virology 163:261–267
Sugiyama M, Minamoto N, Kinjo T, Hirayama N, Sasaki H, Yoshikawa Y, Yamanouchi K (1989) Characterization of monoclonal antibodies against four structural proteins of rinderpest virus. J Gen Virol 70:2605–2613
Ziegler D, Fournier P, Berbers GA, Steuer H, Wiesmuller KH, Fleckenstein B, Schneider F, Jung G, King CC, Muller CP (1996) Protection against measles virus encephalitis by monoclonal antibodies binding to a cystine loop domain of the H protein mimicked by peptides which are not recognized by maternal antibodies. J Gen Virol 77:2479–2489
Sugiyama M, Ito N, Minamoto N, Tanaka S (2002) Identification of immunodominant neutralizing epitopes on the hemagglutinin protein of Rinderpest. Virus J Virol 76:1691–1696
Singh RP, Sreenivasa BP, Dhar P, Shah LC, Bandyopadhyay SK (2004) Development of a monoclonal antibody based competitive-ELISA for detection and titration of antibodies to peste des petits ruminants (PPR) virus. Vet Microbiol 98:3–15
Giraudon P, Wild TF (1985) Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology 144:46–58
Yilma T, Hsu D, Jones L, Owens S, Grubman M, Mebus C, Yamanaka M, Dale B (1988) Protection of cattle against rinderpest with vaccinia virus recombinants expressing the HA or F gene. Science 242:1058–1061
Barrett T, Banyard AC, Diallo A (2006) Molecular biology of the morbilliviruses. In: Barrett T, Pastoret PP, Taylor WP (eds) Rinderpest and peste des petits ruminants: virus plagues of large and small ruminants. Elsevier, United Kingdom, pp 31–67
Brinckmann UG, Bankamp B, Reich A, Meulen VT, Liebert UG (1991) Efficacy of individual measles virus structural proteins in the protection of rats from measles encephalitis. J Gen Virol 72:2491–2500
Principles and methods of validation of diagnostic assays for infectious diseases (2017) OIE—manual of diagnostic tests for aquatic animals. http://www.oie.int/fileadmin/Home/eng/Health_standards/aahm/current/chapitre_validation_diagnostics_assays.pdf. Accessed 05 May 2017
Diallo A, Barret T, Barbron M, Meyer G, Lefevre PC (1994) Cloning of the nucleocapsid protein gene of peste-des-petits-ruminants virus: relationship to other morbilliviruses. J Gen Virol 75:233–237
Couacy-Hymann E, Bodjo SC, Tounkara K, Koffi MY, Ohui AY, Danho T, Bronsvoort BMD (2007) Comparison of two competitive ELISAs for the detection of specific peste-des-petits-rminants antibodies in sheep and cattle populations. Afr J Biotechnol 6:732–736
Graves M, Griffin DE, Johnson RT, Hirsch RL, de Soriano IL, Roedenbeck S, Vaisberg A (1984) Development of antibody to measles virus polypeptides during complicated and uncomplicated measles virus infections. J Virol 49:409–412
Acknowledgements
We wish to thank the following persons and institutions for supplying small ruminants’ sera for the assay validation process: Dr. George Matlho (Botswana Vaccine Institute: gmatlho@bvi.co.bw) for the PPR-negative sera from Botswana, Dr. Bernd Hoffmann (Friedrich-Loeffler-Institut: Bernd.Hoffmann@fli.bund.de) for the PPR-negative sera from Germany, and Dr. Tesfaye Rufael (National Animal Health Diagnostic and Investigation Center of Ethiopia (NAHDIC): rufaelc@yahoo.com) for supplying the sera from vaccinated animals from Ethiopia. PPR post-vaccination sera from Somalia and PPR-negative sera from Malawi were obtained under the projects OSRO/SOM/301/MUL (financially supported by the Food and Agriculture Organization of United Nations-FAO) and Vaccines for the Control of Neglected Animal Diseases in Africa-VACNADA (financed by The European Union-EU), respectively.
Funding
H-based PPR-bELISA research study was funded under the African Union Commission budget.
Author information
Authors and Affiliations
Contributions
BSC designed and supervised the study; generated the monoclonal antibodies and produced the reagents used in the development of the assay, carried out some of the ELISA tests and prepared the manuscript. BJD carried out the ELISA tests and characterised the monoclonal antibodies. NN contributed towards the analysis of the assay data and reviewed the manuscripts. KT contributed towards the design of the study and the analysis of data. KYM carried out the ELISA tests for assay comparisons. CHE participated in the inter-laboratory test study. DM participated in the inter-laboratory test study; GD participated in the inter-laboratory test study; TIBA participated in the inter-laboratory test study; ML participated in the inter-laboratory test study; AD contributed towards the analysis of the results of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. AU-PANVAC is a specialised technical agency of the African Union Commission which has signed a headquarters agreement with the Government of the Federal Democratic Republic of Ethiopia. All laboratory activities are conducted in accordance with the laws and regulations of Ethiopia. Animal manipulations are conducted under the AU-PANVAC Quality Management System.
Additional information
Handling Editor: Diego G. Diel.
Rights and permissions
About this article
Cite this article
Bodjo, S.C., Baziki, JdD., Nwankpa, N. et al. Development and validation of an epitope-blocking ELISA using an anti-haemagglutinin monoclonal antibody for specific detection of antibodies in sheep and goat sera directed against peste des petits ruminants virus. Arch Virol 163, 1745–1756 (2018). https://doi.org/10.1007/s00705-018-3782-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3782-1