Abstract
Human adenoviruses (HAdV) of species B, C, and E (HAdV-B, -C, -E) are frequent causative agents of acute respiratory infections worldwide. No specific analysis has been done on the epidemiological and clinical features of HAdV in pediatric pneumonia in China. Nasopharyngeal aspirates were collected from hospitalized children with pneumonia from June 2009 to May 2014. All samples that tested positive for HAdV were typed by sequencing the hexon and fiber genes. From a total of 3089 samples, 208 (6.7 %) were positive for HAdV, identified as belonging to HAdV-B (186, 89.4 %), HAdV-C (9, 4.3 %) and HAdV-E (1, 0.5 %). HAdV-7 (104, 50.0 %) and HAdV-3 (78, 37.5 %) were the two major types, followed by HAdV-1, HAdV-55 and HAdV-14. There were 87 (41.8 %) single HAdV infections, of which 80 % were HAdV-7 infections. Multivariate analysis showed that single infections with HAdV-7 were associated with a higher prevalence of severe pneumonia. Temporal patterns showed that, except for a simultaneous outbreak of HAdV-3 and HAdV-7 during the years 2010–2011, HAdV-7 and HAdV-3 were alternately predominant, and the dominance shifted to HAdV-3 after 2014. Identification of the predominant HAdV genotypes and their epidemical features is useful for determining preventive strategies. HAdV-7 associated severe pneumonia needs to be considered with high priority in clinical practice.
Similar content being viewed by others
Introduction
Human adenoviruses (HAdVs) are a significant cause of acute respiratory disease (ARD). They are associated with sporadic infection, as well as with community and institutional outbreaks, particularly among military recruits [7]. There are at least 68 recognized HAdV genotypes (http://HAdVwg.gmu.edu/), which are assigned to seven subgroups (A-G) according to their biophysical, biochemical, and genetic characteristics, with marked differences in tissue tropism and clinical manifestations. Depending on the infecting HAdV types, a broad spectrum of clinical illnesses can be observed [13]. The types most commonly associated with respiratory syndromes belong to HAdV species C (HAdV-C1, -C2, -C5, and -C6), HAdV species B, subspecies B1 (HAdV-3 and HAdV-7) and B2 (HAdV-14) [15]. These viruses rarely cause serious or fatal illness in otherwise healthy individuals, but they can cause severe disease in newborn, elderly, or immunocompromised persons [6, 19]. A well-known feature of HAdV is frequent recombination between members of the same species and between members of different species, which has acted as an important force driving the evolution of HAdV genetics [12]. For the emerging new types or recombinant strains, there is strong potential for wide spread and even epidemic outbreaks, partially due to the lack of herd immunity. There is therefore a need for continuous surveillance, with extensive molecular characterization, to determine the prevalence and genetic characteristics of the circulating HAdVs.
The current study was performed to identify the predominant HAdV types associated with pediatric pneumonia and to trace the new genetic variants that might be derived from recombination of different types. Clinical features were also revealed with regard to HAdV genotype and genetic characteristics.
Materials and methods
Study setting and patient recruitment
The current study was performed as part of an ongoing, integrated surveillance for acute respiratory tract infections (ARTI) carried out in the respiratory department of Children’s Hospital of Chongqing Medical University (CHCMU). CHCMU is the largest children’s hospital in southeastern China, serving the population of Sichuan Province and two neighboring provinces, with 110 beds in the respiratory department. The surveillance project was begun in June 2009, and a total of 3487 ARI patients were recruited by the end of May 2014. From the cohort, patients with pneumonia were selected to analyze the epidemiological and clinical features of HAdV infection.
Pneumonia was defined by the presence of patchy alveolar opacities in chest radiographs, in addition to symptoms of cough, dyspnea (lower chest wall indrawing), or tachypnea (in infants, >50–60 breaths/min; in older children, >40 breaths/min). Severe pneumonia was defined as pneumonia plus hypoxemia (maintained SaO2 <92 % in air) or rising respiratory and pulse rates with clinical evidence of respiratory distress and exhaustion with or without raised PaCO2. Underlying diseases included asthma, eczema and tuberculosis.
Detection of HAdV and other respiratory viruses
Nasopharyngeal aspirates (NPA) were collected from the patients upon hospitalization and stored in a −80 °C freezer until they were tested. DNA and RNA were extracted from each specimen using a QIAamp® MinElute Virus Spin Kit (QIAGEN, Hilden, Germany). Molecular assays for HAdV detection were performed using pan primers as described previously [22].The presence of other viral pathogens, including influenza viruses A and B, respiratory syncytial virus (RSV) subtypes A and B, parainfluenza virus (PIV) types 1, 2, 3, and 4, metapneumovirus (MPV), human rhinovirus (HRV), coronavirus (COV), and human bocavirus (HBoV), were tested by RT-PCR or PCR as described previously [6, 19]. Each PCR run included viral DNA or RNA as a positive control and water as a negative control. For samples that were positive by generic HAdV PCR, the entire fiber gene and highly variable regions of hexon gene were amplified and then sequenced as described previously [1, 14].
Statistical analysis
Descriptive statistics were performed for all variables; the continuous variables were summarized as means and standard deviations (SD) or as medians and ranges, and the categorical variables were summarized as frequencies and proportions. To determine the difference between groups, an independent t-test, the χ2 test, Fisher’s exact test, or a nonparametric test was used where appropriate. The logistic regression model was used to examine the potential risk factors for outcomes such as severe pneumonia. Odds ratios (ORs) and their 95 % confidence intervals (CIs) were estimated using maximum-likelihood methods. A two-sided P-value of <.05 was considered to be statistically significant. All analyses were performed using SAS software, version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
Detection and clinical characteristics of HAdV
Over a period of six years, a total of 3089 NPAs (261 from 2009, 782 from 2010, 869 from 2011, 501 from 2012, 464 from 2013 and 212 from 2014) were collected from children aged 1 month to 14 years (median, 9 months) diagnosed as having pneumonia. Of these, 66.8 % (2064) were boys. Overall, HAdV was detected in 6.7 % (208/3089) of the samples, which were identified as HAdV-B (186, 89.4 %), HAdV-C (9, 4.3 %), HAdV-E (1, 0.5 %) and untyped HAdV (12, 5.8 %, not sequenced due to inadequate sample volume). The typed HAdVs were further classified into six genotypes (104 HAdV-7, 78 HAdV-3, 5 HAdV-5, 4 HAdV-1, 4 HAdV-55, and 1 HAdV-4). The HAdV-positive patients were significantly older than the HAdV-negative patients (13 vs. 8 months, P < 0.001) (Table 1). When compared regarding age, the infection rate increased significantly with older age (Cochran-Armitage trend, −6.2, P < 0.001). The highest positive rate (12.0 %) was found in patients of 4–5 years old. The gender distribution was similar between the HAdV-positive group and the negative group as a whole (P = 0.881) (Table 1).
The most commonly seen clinical manifestations of HAdV-positive patients were fever (100.0 %), cough (97.1 %), moist rale (80.8 %) and expectoration (77.9 %), which did not deviate from those of HAdV-negative patients. However, HAdV infection was associated with more-severe pneumonia and more frequent transfer to the intensive care unit in comparison with HAdV-negative patients (P < 0.001 and P = 0.027, respectively) (Table 1).
The HAdV-7 and HAdV-3 were the most prevalent types, accounting for 50.0 % and 37.5 %, respectively of the total HAdV positive samples. The comparison between the two HAdV types revealed that HAdV-7 infection caused more development of expectoration, dyspnea and diarrhea than HAdV-3 infection (all P < 0.05). Among the 104 HAdV-7-infected patients, 53.9 % (56) were diagnosed as having severe pneumonia, significantly higher than the 26.9 % (21/78) among the HAdV-3-infected patients (P < 0.001) (Table 2). HAdV-3 was similarly common among patients with non-severe and severe pneumonia (2.5 % vs. 2.7 %; P = 0.726), whereas HAdV-7 was the dominant type among patients with severe versus non-severe pneumonia (7.2 % vs. 2.1 %; P < 0.001).
HAdV coinfections and clinical characteristics
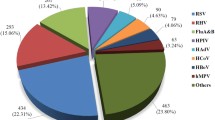
Among the 208 HAdV-infected patients, 87 (41.8 %) had single infections, and 121 (58.2 %) were coinfected with other virus, including RSV (42, 20.2 %), PIV (37, 17.8 %), HRV (30, 14.4 %), HBoV (27, 13 %), influenza virus (16, 7.7 %), COV (4, 1.9 %), and MPV (3, 1.4 %). The children with single HAdV infections were significantly older than those with coinfection (17 months vs. 11 months, P < 0.001). There was also a higher rate of coinfection (68.9 %) in children of 0–6 months, mostly with HAdV and RSV (15/45, 30.0 %).The gender distribution was comparable between the single-infection and coinfection groups (P = 0.498) (Table 1). The HAdV-7 had a significantly higher proportion of single infections (52.9 %) than HAdV-3 (30.8 %, P = 0.003). Severe pneumonia occurred in over 46 % (40/87) of HAdV single infections, among which 80 % (32/40) were HAdV-7 infections.
Coinfection with HAdV and other respiratory virus did not result in a difference in clinical manifestation or severe clinical outcomes. The differences for neutrophils, lymphocyte, hemoglobin and platelet count between HAdV-positive and negative patients attained a significant level, which was not observed between single HAdV infections and coinfections (Table 1).
Severe pneumonia was considered an important dependent variable and analyzed for its risk factors by applying multivariate logistic analysis for HAdV infection in general and for HAdV-3/HAdV-7 specifically. In the HAdV-positive vs. negative model, after adjusting for the effects of age, gender, delay from onset to admission, and coinfection with other viruses, HAdV infection was demonstrated to significantly increase the risk of severe pneumonia (OR, 2.391; 95 % CI, 1.774–3.223). In the HAdV-3 vs. HAdV-7 model, the OR of HAdV-7 for severe pneumonia was 1.318 (95 % CI, 1.119–1.553) compared to HAdV-3 after considering the other effects (Table 3).
Temporal distribution of HAdV
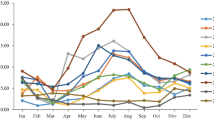
Temporally, HAdV was active all year round but displayed characteristic biennial incidence peaks with alternating low peaks appearing in the winter and summer, respectively (Fig. 1). A simultaneous outbreak of HAdV-3 and HAdV-7 occurred during the years 2010–2011, with the proportion of HAdV-7 increasing significantly to 10.9 % during the winter peak and to 15.7 % during the summer peak in 2011 (P < 0.001). In comparison, the incidence peaks of HAdV-3 in winter and summer were less prominent. The proportion of HAdV-3 remained at levels comparable to those of the other years. After a shift to HAdV-7 since 2010, another round of HAdV-3-dominant circulation emerged after 2014 (Fig. 1).
HAdV infections in pediatric pneumonia cases in Chongqing, China, from June 2009 to May 2014 by month of onset of illness. The bi-monthly HAdV-positive rate is defined as the number of respiratory tract specimens in which HAdV was detected divided by the number of all specimens submitted from pneumonia patients in the corresponding months
Discussion
Pediatric pneumonia caused by viral infection has always been of great public-health concern worldwide because of its high morbidity and mortality. A wide variety of etiological agents has been documented, among which HAdV is thought to be a significant contributor. In China, there have been recent studies investigating the prevalence of HAdV subspecies B1 in respiratory distress, but mostly involving adults. A multicenter surveillance for community-acquired pneumonia that was conducted in northern China between 2010 and 2012 demonstrated HAdV-55 to be the predominant genotype, followed by HAdV-7, HAdV-3, HAdV-14 and HAdV-50. This study, however, provided no data on children [2]. In Taiwan, China, a large community outbreak of HAdV in 2011 was shown to be predominantly associated with HAdV-3, although HAdV-7 began to show an increased circulation [10, 20]. Similarly, in some studies and outbreaks in which respiratory adenoviruses were identified and typed, in Guangzhou during 2010 to 2011, in Hangzhou during 2011 [21] and in Shaanxi [18], the most common HAdV type was shown to be HAdV-3 [8]. The prevalence of HAdV and the circulating genotypes in pediatric respiratory infection have been investigated in two recent studies. In Lanzhou, from 2006 to 2009, HAdV infections represented 6.33 % of ARTI of viral etiology in children, and among all HAdV genotypes, HAdV-3 was most frequently detected, followed by HAdV-11 and other types [9]. Our previous study performed in Chongqing during 2009–2012 identified the emergence of HAdV-55 in pediatric patients with ARTI, but there was no comprehensive analysis of all HAdV genotypes in the local region [11]. In most of the above-mentioned studies, no specific analysis was done of the epidemic and clinical features of HAdV in cases of pediatric pneumonia. HAdV coinfection with another respiratory virus was rarely determined, and differentiation of HAdV-associated clinical manifestations from other viral respiratory viral infection was not mentioned.
Here, in a prolonged surveillance study, we have identified HAdV-7 and HAdV-3 as the two major genotypes causing pediatric pneumonia in southeastern China. These two genotypes alternate as the predominant cause of pediatric pneumonia. Although the seasonality of HAdV-related pneumonia is less distinct, the notable increase in circulation of HAdV-7 between 2010 and 2011 might represent an undetected outbreak in local children, which, however, might have been undiagnosed or misdiagnosed. A recent report of an HAdV-7-associated outbreak in a military camp in Shaanxi, China [23], could likewise have been caused by the predominant circulation of HAdV-7 in the local area, as demonstrated in the current study. With the advent of HAdV-3 dominance since 2014 in Chongqing, we propose a high potential of outbreak events caused by HAdV-3 in the local area.
In the United States, the HAdV-7 prototype strain accounted for two-thirds of HAdV-7 isolates from 1966 to 2000 [4]. Changes in serotypes and genome types among geographic regions underscore the potential for new strains to evolve and replace existing strains. In the United States and southern Ontario from 2004 to 2006, HAdV-3 accounted for 34.6 % of HAdV respiratory tract infection in civilians and 2.6 % among military trainees, while HAdV-7 accounted for only 5/581 (0.9 %) of clinical HAdV respiratory isolates in military facilities and 48/1653 (2.9 %) of isolates in civilian settings [5] and 0.7 % in Zagreb County [17]. By contrast, HAdV-7 has been a prominent cause of FRI in Asia. Especially in the current study, we observed a high proportion of HAdV-7 and HAdV-3 in pediatric pneumonia, which was similar to that in Beijing, China [3]. The predominant serotypes differ among different countries or regions, and they change over time [13]. This also might reflect more-severe clinical disease in the current patients than in those of previous studies [9, 10, 16], where outpatients with ARD instead of pneumonia were recruited.
We also found the clinical syndromes and symptoms of both HAdV single infection and HAdV coinfection to be largely nonspecific and less differentiated from those of HAdV-negative patients. As a result, ascertaining HAdV infection based on the clinical manifestations is challenging. However, we found that pneumonia caused by HAdV infection was more severe than that caused by other viral infections, with over half of the cases of HAdV-associated pneumonia causes by HAdV-7. Coinfection with other respiratory viruses, on the other hand, did not lead to more-severe disease outcome. These findings have the clinical implication that in patients with severe pneumonia, the contribution of HAdV, especially HAdV-7, should be considered with high priority, regardless of whether another respiratory pathogen has been detected.
Because we did not conduct a seroconversion study, the exact role of HAdVs by themselves in clinical manifestations and pathogenicity remains to be studied in the future.
In conclusion, the identification of the predominant HAdV genotypes and their temporal pattern could help to achieve a better prediction of seasonal activity, guiding timely preventive strategies if a vaccine is available. The clinical pictures and clinical outcomes of HAdV infection alone and in combination with other pathogens might help to inform clinical practice.
References
Biere B, Schweiger B (2010) Human adenoviruses in respiratory infections: sequencing of the hexon hypervariable region reveals high sequence variability. J Clin Virol 47:366–371
Cao B, Huang GH, Pu ZH, Qu JX, Yu XM, Zhu Z, Dong JP, Gao Y, Zhang YX, Li XH, Liu JH, Wang H, Xu Q, Li H, Xu W, Wang C (2014) Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest 145:79–86
Deng J, Qian Y, Zhao LQ, Zhu RN, Sun Y, Tian R (2013) Identification and typing of adenovirus from acute respiratory infections in pediatric patients in Beijing from 2003 to 2012. Chin J Virol 29:615–620
Erdman DD, Xu W, Gerber SI, Gray GC, Schnurr D, Kajon AE, Anderson LJ (2002) Molecular epidemiology of adenovirus type 7 in the United States, 1966-2000. Emerg Infect Dis 8:269–277
Gray GC, McCarthy T, Lebeck MG, Schnurr DP, Russell KL, Kajon AE, Landry ML, Leland DS, Storch GA, Ginocchio CC, Robinson CC, Demmler GJ, Saubolle MA, Kehl SC, Selvarangan R, Miller MB, Chappell JD, Zerr DM, Kiska DL, Halstead DC, Capuano AW, Setterquist SF, Chorazy ML, Dawson JD, Erdman DD (2007) Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004-2006. Clin Infect Dis 45:1120–1131
Gunson RN, Collins TC, Carman WF (2005) Real-time RT-PCR detection of 12 respiratory viral infections in four triplex reactions. J Clin Virol 33:341–344
Hall CE, Brandt CD, Frothingham TE, Spigland I, Cooney MK, Fox JP (1971) The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. IX. A comparison of infections with several respiratory pathogens in New York and New Orleans families. Am J Epidemiol 94:367–385
Han G, Niu H, Zhao S, Zhu B, Wang C, Liu Y, Zhang M, Yang S, Liu F, Wan C, Zhang Q (2013) Identification and typing of respiratory adenoviruses in Guangzhou, Southern China using a rapid and simple method. Virol Sin 28:103–108
Jin Y, Zhang RF, Xie ZP, Yan KL, Gao HC, Song JR, Yuan XH, Hou YD, Duan ZJ (2013) Prevalence of adenovirus in children with acute respiratory tract infection in Lanzhou, China. Virol J 10:271
Lai CY, Lee CJ, Lu CY, Lee PI, Shao PL, Wu ET, Wang CC, Tan BF, Chang HY, Hsia SH, Lin JJ, Chang LY, Huang YC, Huang LM, Taiwan Pediatric Infectious Disease Alliance (2013) Adenovirus serotype 3 and 7 infection with acute respiratory failure in children in Taiwan, 2010–2011. PLoS ONE 8:e53614
Lu QB, Tong YG, Wo Y, Wang HY, Liu EM, Gray GC, Liu W, Cao WC (2014) Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009-2012. Influenza Other Respir Viruses 8:302–308
Lukashev AN, Ivanova OE, Eremeeva TP, Iggo RD (2008) Evidence of frequent recombination among human adenoviruses. J Gen Virol 89:380–388
Lynch JP 3rd, Fishbein M, Echavarria M (2011) Adenovirus. Semin Respir Crit Care Med 32:494–511
Madisch I, Harste G, Pommer H, Heim A (2005) Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J Virol 79:15265–15276
Moura PO, Roberto AF, Hein N, Baldacci E, Vieira SE, Ejzenberg B, Perrini P, Stewien KE, Durigon EL, Mehnert DU, Harsi CM (2007) Molecular epidemiology of human adenovirus isolated from children hospitalized with acute respiratory infection in Sao Paulo, Brazil. J Med Virol 79:174–181
Rojas LJ, Jaramillo CA, Mojica MF, Escalante MP, Delgado P (2012) Molecular typing of adenovirus circulating in a Colombian paediatric population with acute respiratory infection. Epidemiol Infect 140:818–822
Tabain I, Ljubin-Sternak S, Cepin-Bogovic J, Markovinovic L, Knezovic I, Mlinaric-Galinovic G (2012) Adenovirus respiratory infections in hospitalized children: clinical findings in relation to species and serotypes. Pediatr Infect Dis J 31:680–684
Tang L, Wang L, Tan X, Xu W (2011) Adenovirus serotype 7 associated with a severe lower respiratory tract disease outbreak in infants in Shaanxi Province, China. Virol J 8:23
Tiveljung-Lindell A, Rotzen-Ostlund M, Gupta S, Ullstrand R, Grillner L, Zweygberg-Wirgart B, Allander T (2009) Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses. J Med Virol 81:167–175
Tsou TP, Tan BF, Chang HY, Chen WC, Huang YP, Lai CY, Chao YN, Wei SH, Hung MN, Hsu LC, Lu CY, Shao PL, Mu JJ, Chang LY, Liu MT, Unknown Pathogen Discovery/Investigation Group, Huang LM (2012) Community outbreak of adenovirus, Taiwan, 2011. Emerg Infect Dis 18:1825–1832
Xie L, Yu XF, Sun Z, Yang XH, Huang RJ, Wang J, Yu A, Zheng L, Yu MC, Hu XW, Wang BM, Chen J, Pan JC, Liu SL (2012) Two adenovirus serotype 3 outbreaks associated with febrile respiratory disease and pharyngoconjunctival fever in children under 15 years of age in Hangzhou, China, during 2011. J Clin Microbiol 50:1879–1888
Xu W-H, Mcdonough MC, Erdman DD (2000) Species-specific identification of human adenoviruses by a multiplex PCR assay. J Clin Microbiol 38:4114–4120
Yu P, Ma C, Nawaz M, Han L, Zhang J, Du Q, Zhang L, Feng Q, Wang J, Xu J (2013) Outbreak of acute respiratory disease caused by human adenovirus type 7 in a military training camp in Shaanxi, China. Microbiol Immunol 57:553–560
Acknowledgments
This study was supported by the China Mega-Project for Infectious Diseases grant (2013ZX10004-202), the Natural Science Foundation of China (81222037) and the Youth Talent Support Program by School of Public Health, Peking University.
Conflict of interest
Regarding this report, the authors do not have any commercial or other associations that would be considered a conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Y. Wo and Q.-B. Lu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wo, Y., Lu, QB., Huang, DD. et al. Epidemical features of HAdV-3 and HAdV-7 in pediatric pneumonia in Chongqing, China. Arch Virol 160, 633–638 (2015). https://doi.org/10.1007/s00705-014-2308-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-014-2308-8