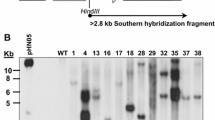
Abstract
Classical swine fever (CSF), caused by the CSF virus (CSFV), is a highly contagious disease in pigs. In Korea, vaccination using a live-attenuated strain (LOM strain) has been used to control the disease. However, parenteral vaccination using a live-attenuated strain still faces a number of problems related to storage, cost, injection stress, and differentiation of CSFV infected and vaccinated pigs. Therefore, two kinds of new candidates for oral vaccination have been developed based on the translation of the E2 gene of the SW03 strain, which was isolated from an outbreak of CSF in 2002 in Korea, in transgenic rice calli (TRCs) from Oriza sativa L. cv. Dongjin to express a recombinant E2 protein (rE2-TRCs). The expression of the recombinant E2 protein (rE2) in rE2-TRCs was confirmed using Northern blot, SDS-PAGE, and Western blot analysis. Immune responses to the rE2-TRC in mice and pigs were investigated after oral administration. The administration of rE2-TRCs increased E2-specific antibodies titers and antibody-secreting cells when compared to animals receiving the vector alone (p < 0.05 and p < 0.01). In addition, mice receiving rE2-TRCs had a higher level of CD8+ lymphocytes and Th1 cytokine immune responses to purified rE2 (prE2) in vitro than the controls (p < 0.05 and p < 0.01). Pigs receiving rE2-TRCs also showed an increase in IL-8, CCL2, and the CD8+ subpopulation in response to stimulation with prE2. These results suggest that oral administration of rE2-TRCs can induce E2-specific immune responses.





Similar content being viewed by others
References
Wengler G, Baradley DW, Collett MS, Heinz FX, Schlesinger RW, Strauss JH (1995) Family flaviviridae. In: Murphy FA, Fauquet CM, Bishop DHL, Ghabrial SA, Jarvis AW, Martelli GP, Mayo MO, Summer MD (eds) Virus taxonomy. Classification and nomenclature of viruses. Sixth Report of the International Committee on Taxonomy of Viruses. Springer, Wien New York, pp 415–427
Dong XN, Chen YH (2007) Marker vaccine strategies and candidate CSFV marker vaccines. Vaccine 25:205–230. doi:10.1016/j.vaccine.2006.07.033
Risatti GR, Holinka LG, Carrillo C, Kutish GF, Lu Z, Tulman ER, Femandez Sainz I, Borca MV (2006) Identification of a novel virulence determinant within the E2 structural glycoprotein of classical swine fever virus. Virology 355:94–101. doi:10.1016/j.virol.2006.07.005
König M, Lengsfeld T, Pauly T, Stark R, Thiel H (1995) Classical swine fever virus: independent induction of protective immunity by two structural glycoproteins. J Virol 69:6479–6486
Rümenapf T, Stark R, Meyers G, Thiel HJ (1991) Structural proteins of hog cholera virus expressed by vaccinia virus: further characterization and induction of protective immunity. J Virol 65:589–597
Weiland E, Stark R, Haas B, Rümenapf T, Meyers G, Thiel HJ (1990) Pestivirus glycoprotein which induces neutralizing antibodies forms part of a disulfide-linked heterodimer. J Virol 64:3563–3569
Sakoda Y, Wakamoto H, Tamura T, Nomura T, Naito M, Aoki H, Morita H, Kida H, Fukusho A (2012) Development and evaluation of indirect enzyme-linked immunosorbent assay for a screening test to detect antibodies against classical swine fever virus. Jpn J Vet Res 60:85–94
Edwards S, Fukusho A, Lefevre PC, Lipowski A, Pejsak Z, Roehe P, Westergaard J (2000) Classical swine fever: the global situation. Vet Microbiol 73:103–119. doi:10.1016/S0378-1135(00)00138-3
Kim B, Song JY, Tark DS, Lim SI, Choi EJ, Kim J, Park CK, Lee BY, Wee SH, Bae YC, Kwon JH, Kang WC, Kim TY, Kim JH, Lee JH, Kang MI (2008) Feed contaminated with classical swine fever vaccine virus (LOM strain) can induce antibodies to the virus in pigs. Vet Rec 162:12–17. doi:10.1136/vr.162.1.12
Song J, Lim S, Jeoung H, Choi E, Hyun B, Kim B, Kim J, Shin Y, Kim J, Joo H (2013) Prevalence of classical swine fever virus in domestic pigs in South Korea: 1999–2011. Transbound Emerg Dis 60:546–551. doi:10.1111/j.1865-1682.2012.01371.x
Milicevic V, Dietze K, Plavsic B, Tikvicki M, Pinto J, Depner K (2013) Oral vaccination of backyard pigs against classical swine fever. Vet Microbiol 163:167–171. doi:10.1016/j.vetmic.2012.12.005
van Oirschot J (2003) Vaccinology of classical swine fever: from lab to field. Vet Microbiol 96:367–384. doi:10.1016/j.vetmic.2003.09.008
Moennig V (2000) Introduction to classical swine fever: virus, disease and control policy. Vet Microbiol 73:93–102. doi:10.1016/S0378-1135(00)00137-1
Sato U, Nishimura Y, Hanaki T, Nobuto K (1964) Attenuation of hog cholera virus by means of continuous cell-virus propagation (CCVP) method. Arch Gesamte Virusforsch 14:394–403
Graham SP, Everett HE, Johns HL, Haines FJ, Rocca SAL, Khatri M, Wright IK, Drew T, Crooke HR (2010) Characterisation of virus-specific peripheral blood cell cytokine responses following vaccination or infection with classical swine fever viruses. Vet Microbiol 142:34–40. doi:10.1016/j.vetmic.2009.09.040
Moormann RJ, Bouma A, Kramps JA, Terpstra C, De Smit HJ (2000) Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet Microbiol 73:209–219. doi:10.1016/S0378-1135(00)00146-2
van Zijl M, Wensvoort G, De Kluyver E, Hulst M, van der Gulden H, Gielkens A, Berns A, Moormann R (1991) Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol 65:2761–2765
Feliziani F, Blome S, Petrini S, Giammarioli M, Iscaro C, Severi G, Convito L, Pietschmann J, Beer M, De Mia GM (2014) First assessment of classical swine fever marker vaccine candidate CP7_E2alf for oral immunization of wild boar under field conditions. Vaccine 32:2050–2055. doi:10.1016/j.vaccine.2014.02.006
Streatfield SJ, Howard JA (2003) Plant-based vaccines. Int J Parasitol 33:479–493. doi:10.1016/S0020-7519(03)00052-3
Streatfield S (2006) Mucosal immunization using recombinant plant-based oral vaccines. Methods 38:150–157. doi:10.1016/j.ymeth.2005.09.013
Catmull J, Wilson ME, Kirchhoff LV, Metwali A, Donelson JE (1999) Induction of specific cell-mediated immunity in mice by oral immunization with Salmonella expressing Onchocerca volvulus glutathione S-transferase. Vaccine 17:31–39. doi:10.1016/S0264-410X(98)00147-9
Gomez E, Zoth SC, Carrillo E, Roux ME, Berinstein A (2008) Mucosal immunity induced by orally administered transgenic plants. Immunobiology 213:671–675. doi:10.1016/j.imbio.2008.02.002
Hammond J, Jansen E, Morrissy C, Williamson M, Hodgson A, Johnson M (2001) Oral and sub-cutaneous vaccination of commercial pigs with a recombinant porcine adenovirus expressing the classical swine fever virus gp55 gene. Arch Virol 146:1787–1793. doi:10.1007/s007050170064
Hulst MM, Westra DF, Wensvoort G, Moormann RJM (1993) Glycoprotein E1 of hog cholera virus expressed in insect cells protects swine from hog cholera. J Virol 67:5435–5442
Legocki AB, Miedzinska K, Czaplińska M, Płucieniczak A, Wędrychowicz H (2005) Immunoprotective properties of transgenic plants expressing E2 glycoprotein from CSFV and cysteine protease from Fasciola hepatica. Vaccine 23:1844–1846. doi:10.1016/j.vaccine.2004.11.015
Marconi G, Albertini E, Barone P, Marchis FD, Lico C, Marusic C, Rutili D, Veronesi F, Porceddu A (2006) In planta production of two peptides of the classical swine fever virus (CSFV) E2 glycoprotein fused to the coat protein of potato virus X. BMC biotechnol 6:29. doi:10.1186/1472-6750-6-29
Sánchez O, Barrera M, Rodríguez MP, Frías MT, Figueroa NE, Naranjo P, Montesino R, Farnos O, Castell S, Venereo A (2008) Classical swine fever virus E2 glycoprotein antigen produced in adenovirally transduced PK-15 cells confers complete protection in pigs upon viral challenge. Vaccine 26:988–997. doi:10.1016/j.vaccine.2007.11.014
Yuki Y, Tokuhara D, Nochi T, Yasuda H, Mejima M, Kurokawa S, Takahashi Y, Kataoka N, Nakanishi U, Hagiwara Y (2009) Oral MucoRice expressing double-mutant cholera toxin A and B subunits induces toxin-specific neutralising immunity. Vaccine 27:5982–5988. doi:10.1016/j.vaccine.2009.07.071
Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T (2007) Rice-based mucosal vaccine as a global strategy for cold-chain-and needle-free vaccination. Proc Natl Acad Sci 104:10986–10991. doi:10.1073/pnas.0703766104
Sung JH, Kang ML, Lee WJ, Shin MK, Lim SI, Kim BH, Song JY, Yoo HS (2011) Improved sero-monitoring assay for classical swine fever (CSF) using the recombinant E2 protein of a recent Korean isolate. Res Vet Sci 90:329–335. doi:10.1016/j.rvsc.2010.06.003
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. doi:10.1016/0378-1119(89)90358-2
Shin YJ, Hong SY, Kwon TH, Jang YS, Yang MS (2003) High level of expression of recombinant human granulocyte-macrophage colony stimulating factor in transgenic rice cell suspension culture. Biotechnol Bioeng 82:778–783. doi:10.1002/bit.10635
Kim SH, Seo KW, Kim J, Lee KY, Jang YS (2010) The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol 185:5787–5795. doi:10.4049/jimmunol.0903184
Yasuda H, Hayashi Y, Jomori T, Takaiwa F (2006) The correlation between expression and localization of a foreign gene product in rice endosperm. Plant Cell Physiol 47:756–763. doi:10.1093/pcp/pcj049
Chen L, Marmey P, Taylor NJ, Brizard JP, Espinoza C, D’Cruz P, Huet H, Zhang S, de Kochko A, Beachy RN, Fauquet CM (1998) Expression and inheritance of multiple transgenes in rice plants. Nat Biotechnol 16:1060–1064
Shin YJ, Lee NJ, Kim J, An XH, Yang MS, Kwon TH (2010) High-level production of bioactive heterodimeric protein human interleukin-12 in rice. Enzyme Microb Technol 46:347–351. doi:10.1016/j.enzmictec.2009.12.011
Chu CC (1975) Establishment of an efficient medium for another culture of rice through comparative experiments on the nitrogen sources. Sci Sin 18:659–668
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Chuang CF, Meyerowitz EM (2000) Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci USA 97:4985–4990. doi:10.1073/pnas.060034297
Cha SB, Rayamajhi N, Kang ML, Lee WJ, Shin MK, Yoo HS (2010) Comparative study of gamma interferon production in mice immunized with outer membrane proteins and whole bacteria of Brucella abortus. Jpn J Infect Dis 10:49–51
MacDonald T, Carter P (1982) Isolation and functional characteristics of adherent phagocytic cells from mouse Peyer’s patches. Immunology 45:769–774
Piriou L, Chevallier S, Hutet E, Charley B, Le Potiera MF, Albina E (2003) Humoral and cell-mediated immune responses of d/d histocompatible pigs against classical swine fever (CSF) virus. Vet Res 34:389–404. doi:10.1051/vetres:2003013
Liu S, Tu C, Wang C, Yu X, Wu J, Guo S, Shao M, Gong Q, Zhu Q, Kong X (2006) The protective immune response induced by B cell epitope of classical swine fever virus glycoprotein E2. J Virol Methods 134:125–129. doi:10.1016/j.jviromet.2005.12.008
Reimann I, Depner K, Trapp S, Beer M (2004) An avirulent chimeric Pestivirus with altered cell tropism protects pigs against lethal infection with classical swine fever virus. Virology 322:143–157. doi:10.1016/j.virol.2004.01.028
Devriendt B, De Geest BG, Cox E (2011) Designing oral vaccines targeting intestinal dendritic cells. Expert Opin Drug Deliv 8:467–483. doi:10.1517/17425247.2011.561312
Staats HF, Jackson RJ, Marinaro M, Takahashi I, Kiyono H, McGhee JR (1994) Mucosal immunity to infection with implications for vaccine development. Curr Opin Immunol 6:572–583. doi:10.1016/0952-7915(94)90144-9
Zeng F, Chow KYC, Hon CC, Law KM, Yip CW, Chan KH, Peiris JSM, Leung FCC (2004) Characterization of humoral responses in mice immunized with plasmid DNAs encoding SARS-CoV spike gene fragments. Biochem Biophys Res Commun 315:1134–1139. doi:10.1016/j.bbrc.2004.01.166
Tizard IR (2004) Veterinary immunology: an introduction, 7th edn. Patricia Tannian, Philadelphia
Brayden DJ, Jepson MA, Baird AW (2005) Keynote review: intestinal Peyer’s patch M cells and oral vaccine targeting. Drug Discov Today 10:1145–1157. doi:10.1016/S1359-6446(05)03536-1
Riordan SM, Skinner N, Nagree A, McCallum H, McIver CJ, Kurtovic J, Hamilton JA, Bengmark S, Williams R, Visvanathan K (2003) Peripheral blood mononuclear cell expression of toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology 37:1154–1164. doi:10.1053/jhep.2003.50180
Pauly T, Elbers K, König M, Lengsfeld T, Saalmüller A, Thiel HJ (1995) Classical swine fever virus-specific cytotoxic T lymphocytes and identification of a T cell epitope. J Gen Virol 76:3039–3049
Kourilsky P, Truffa-Bachi P (2001) Cytokine fields and the polarization of the immune response. Trends Immunol 22:502–509. doi:10.1016/S1471-4906(01)02012-9
Toledo JR, Barrera M, Farnós O, Gómez S, Rodríguez MP, Aguero F, Ormazabal V, Parra NC, Suárez L, Sánchez O (2010) Human αIFN co-formulated with milk derived E2-CSFV protein induce early full protection in vaccinated pigs. Vaccine 28:7907–7914. doi:10.1016/j.vaccine.2010.09.073
Zhao HP, Sun JF, Li N, Sun Y, Wang Y, Qiu HJ (2009) Prime-boost immunization using alphavirus replicon and adenovirus vectored vaccines induces enhanced immune responses against classical swine fever virus in mice. Vet Immunol Immunopathol 131:158–166. doi:10.1016/j.vetimm.2009.04.003
Schirmbeck R, Reimann J (2001) Modulation of gene-gun-mediated Th2 immunity to hepatitis B surface antigen by bacterial CpG motifs or IL-12. Intervirology 44:115–123. doi:10.1159/000050038
Wienhold D, Armengol E, Marquardt A, Marquardt C, Voigt H, Büttner M, Saalmüller A, Pfaff E (2005) Immunomodulatory effect of plasmids co-expressing cytokines in classical swine fever virus subunit gp55/E2-DNA vaccination. Vet Res 36:571–587. doi:10.1051/vetres:2005019
Lummus ZL, Alam R, Bernstein JA, Bernstein DI (1998) Diisocyanate antigen–enhanced production of monocyte chemoattractant protein-1, IL-8, and tumor necrosis factor-α by peripheral mononuclear cells of workers with occupational asthma. J Allergy Clin Immunol 102:265–274. doi:10.1016/S0091-6749(98)70095-8
Bensaude E, Turner JL, Wakeley PR, Sweetman DA, Pardieu C, Drew TW, Wileman T, Powell PP (2004) Classical swine fever virus induces proinflammatory cytokines and tissue factor expression and inhibits apoptosis and interferon synthesis during the establishment of long-term infection of porcine vascular endothelial cells. J Gen Virol 85:1029–1037. doi:10.1099/vir.0.19637-0
Renson P, Le Dimna M, Gabriel C, Levai R, Blome S, Kulcsar G, Koenen F, Le Potier M (2014) Cytokine and Immunoglobulin isotype profiles during CP7_E2alf vaccination against a challenge with the highly virulent Koslov strain of Classical swine fever virus. Res Vet Sci 96:389–395. doi:10.1016/j.rvsc.2014.01.002
Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29:313–326. doi:10.1089/jir.2008.0027
Rynda A, Maddaloni M, Mierzejewska D, Ochoa-Repáraz J, Maślanka T, Crist K, Riccardi C, Barszczewska B, Fujihashi K, McGhee JR, Pascual DW (2008) Low-dose tolerance is mediated by the microfold cell ligand, reovirus protein σ1. J Immunol 180:5187–5200. doi:10.4049/jimmunol.180.8.5187
Weiner HL, da Cunha AP, Quintana F, Wu H (2011) Oral tolerance. Immunol Rev 241:241–259. doi:10.1111/j.1600-065X.2011.01017.x
Ogra PL, Faden H, Welliver RC (2001) Vaccination strategies for mucosal immune responses. Clin Microbiol Rev 14:430–445. doi:10.1128/CMR.14.2.430-445.2001
Acknowledgments
This study was supported by grant ARPC 307011-5, the BK21 program for Veterinary Science, and the Research Institute of Veterinary Science, Seoul National University, Korea.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, M., Shin, Y.J., Kim, J. et al. Induction of immune responses in mice and pigs by oral administration of classical swine fever virus E2 protein expressed in rice calli. Arch Virol 159, 3219–3230 (2014). https://doi.org/10.1007/s00705-014-2182-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-014-2182-4