Abstract
The objective of this study was to obtain a better understanding of the effects of meteorological factors on the prevalence and seasonality of common respiratory viruses in China, which has a subtropical climate. A retrospective study was conducted by identifying children admitted to a hospital with acute respiratory infections due to seven common viruses between January 2001 and December 2011. A total of 42,104 nasopharyngeal samples were tested for respiratory syncytial virus (RSV), influenza A and B viruses (IV-A and IV-B), parainfluenza viruses 1-3 (PIV-1, PIV-2, PIV-3), and adenovirus (ADV) by direct immunofluorescence assay. Meteorological data were obtained from Suzhou Weather Bureau. Correlations of viral prevalence with meteorological factors were evaluated using Spearman rank correlation and partial correlation. Multivariate time-series analysis including an autoregressive integrated moving average (ARIMA) model and generalized linear Poisson models was conducted to study the effect of meteorological factors on the prevalence of respiratory virus infection. RSV and IV-A activity showed distinctive winter peak, whereas PIV-3 and ADV peaked in the summer. Incidence of RSV was correlated with low environmental temperature, and PIV-3 only with high temperature. IV-A activity was correlated with both low temperature and high relative humidity. ADV activity was correlated with high total rainfall. In the ARIMA model, RSV-associated hospitalizations were predictable, and the monthly number of RSV cases decreased by 11.25 % (95 % CI: 5.34 % to 16.79 %) for every 1 °C increase in the average temperature. Seasonality of certain respiratory virus may be explained by meteorological influences. The impact of meteorological factors on the prevalence of RSV may be useful for predicting the activity of this virus.
Similar content being viewed by others
Introduction
In recent years, the rapid spread of respiratory viruses has gained great attention among scientists, especially with the frequent H5N1 outbreaks in 2003 and the H1N1 pandemic in 2009. How a seasonal epidemic of respiratory viruses starts and is sustained is not yet clearly known. Factors such as viral mutation, host susceptibility and seasonal behavior as well as effects of meteorological factors on the survival and spread of infectious pathogen in the environment may contribute to an epidemic and the seasonality of respiratory viruses [1–3]. Among these factors, meteorological factors have the greatest potential to play key roles in epidemics and seasonal respiratory viruses.
Few studies have investigated the possible relationships between meteorological factors and respiratory viruses, especially with respiratory syncytial virus (RSV) [4–6] and influenza virus [7–9]. In subtropical and temperate climates, the epidemic of RSV peaks during the winter in both the northern and southern hemispheres [10], while the epidemic of influenza has had a clear and consistent seasonality, with an annual winter peak lasting 5-10 weeks during the November-March period in the northern hemisphere, and during May-September in the southern hemisphere [11]. In tropical climates, RSV can cause infections throughout the year, with a small peak during the rainy season [4], while influenza A (IV-A) virus predominates during the months of November-January and June-July and influenza B (IV-B) virus peaks during March-April and July-December [12]. These data support the influence of different climates on the epidemiology of respiratory viruses.
However, there is lack of information on relationships between respiratory virus activity and meteorological factors. Moreover, in different countries and geographic regions, the meteorological factors may also vary. Studies performed in Hong Kong [13, 14] showed that the incidence of IV-A and RSV increased with high relative humidity, while that of IV-B decreased with high temperatures. Clear information on the relationship between respiratory virus incidence and meteorological factors such as temperature, relative humidity, sunshine, rainfall, and wind velocity in subtropical countries is scarce.
The purpose of this study was to evaluate the effects of meteorological factors on the prevalence and seasonality of common respiratory viruses in China, which has a subtropical climate.
Materials and methods
Study design
In this retrospective study, subjects were <14 years in age and hospitalized for acute respiratory infections (ARIs) between January 1, 2001 and December 31, 2011 in the Respiratory Department at the Children’s Hospital affiliated to Soochow University. The demographic and clinical characteristics of all subjects were collected for analysis. This tertiary teaching hospital in Suzhou is the only hospital that provides special care to pediatric patients. This study was conducted with the approval of the Institutional Human Ethical Committee of Soochow University.
Surveillance for common respiratory viruses
Samples of nasopharyngeal aspirates from children hospitalized with ARIs were obtained according to a standard protocol described previously [15]. These samples were obtained from each patient within 24 h of admission by introducing a sterile plastic catheter into the lower pharynx via the nasal cavity. Samples were then analyzed for seven common viruses, including RSV, IV-A and IV-B, parainfluenza viruses 1, 2, 3 (PIV-1, 2, 3), and adenovirus (ADV), using commercial slide-based assays with virus-specific fluorescence-labeled monoclonal antibodies (Light Diagnostics Respiratory Viral Screen DFA, Chemicon International, USA, from 2001-2005; D3 UltraTM DFA Respiratory Virus Screening & ID Kit, Diagnostic Hybrids Inc., USA, from 2006-2011) and ultraviolet light microscopy. The direct fluorescence assay has a sensitivity of 86 %, which is higher than that of RT-PCR [16]. The daily sum of virus-positive cases was routinely collated and recorded with the monthly rate for each respiratory virus.
Meteorological data
Meteorological data for Suzhou, including daily mean temperature (°C), mean relative humidity (%), total rain (mm), total hours of sunshine (h), and mean wind velocity (m/s), were obtained from Suzhou Weather Bureau, which is located 8 km away from the hospital. Meteorological data were obtained hourly, and average daily values were calculated. Monthly means were calculated using the daily means for temperature, relative humidity, and wind velocity. Total rain and hours of sunshine were calculated as a total measurement for the month.
Statistical analysis
The percentage of samples positive for respiratory viruses was analyzed for each month. We used monthly series rather than the weekly series to simplify the amount of data and to allow the associations between virus prevalence and climate variables, if any, to be more easily detectable.
Numerical data were compared using the chi-square test. Correlations of different viral prevalence with meteorological factors were evaluated using Spearman rank correlation. Because of colinearity between meteorological factors, associations between meteorological factors and viral prevalence were also analyzed using partial correlation.
Multiple time series analysis based on autoregressive integrated moving average (ARIMA) models that predict the value of the target variable with a linear function of lag values (autoregressive part) plus an effect from recent random shock values (moving average part) was carried out using an expert modeling procedure with SPSS software, and this could automatically select the most suitable ARIMA model for the influence of meteorological factors on the prevalence of viral ARIs in hospitalized children. Because of the flexibility of the ARIMA models (Box and Jenkins models) for controlling the autocorrelation of time series data, the Box-Ljung test was used to test the null hypothesis that the autocorrelations of the residual time series were equal to zero. For each respiratory virus, an ARIMA model for monthly prevalence was developed following the Box-Jenkins method.
For the final goal of predicting the prevalence of RSV infections on the basis of meteorological data, another ARIMA model was used for the time period between January 2001 and December 2006 (estimation period) as described previously [17], and it was evaluated by comparing the predicted versus the observed prevalence of viral ARIs during the period between January 2007 and December 2011 (i.e., the evaluation period). The R2 autoregression coefficient was calculated for the model. Using a generalized linear Poisson model based on the monthly number of RSV cases, we then examined relative risk and lag effects in greater detail by fitting linear unconstrained distributed lag models [18] that included temperature terms at each lag period that could be as long as 8 months.
All statistical tests were 2-tailed, and P-values <0.05 were considered statistically significant. All analyses were performed using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Description of demographic and clinical characteristics of the children in the study, virus seasonality, and cyclical periodicity
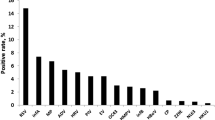
The demographic and clinical characteristics of the children included in this study are shown in Table 1. From January 2001 to December 2011, the average numbers of samples tested for common respiratory viruses per month are shown in Table 2. The number of respiratory samples received for testing increased following the 2003 severe acute respiratory syndrome outbreaks. A total of 42,104 nasopharyngeal samples were tested at Suzhou Children’s Hospital in patients with ARIs. Overall, at least one pathogen was identified in 11,785 cases, and 12,603 viruses were detected using a direct fluorescence assay. RSV was the most common virus detected (19.9 %), followed by PIV-3 (3.7 %), IV-A (3.3 %), ADV (1.4 %), PIV-1 (0.9 %), IV-B (0.6 %), and PIV-2 (0.2 %).
With regard to the seasonal pattern for the most common respiratory viruses, RSV and IV-A activity showed a distinctive winter peak, ADV showed a spring/summer peak, and PIV-3 was frequently detected in summer (Table 3).
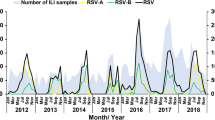
In terms of cyclical periodicity, the monthly prevalence of the four most common respiratory viruses is shown in Figure 1A-D. RSV activity displayed a biennial pattern with a larger winter peak alternating with a smaller one during the following year. IV-A and PIV-3 activity showed an annually recurring pattern, and ADV activity was detected throughout the year. Increased RSV and IV-A activity was observed with low environmental temperature, and increased PIV-3 activity was observed with high environmental temperature (Fig. 1).
Monthly distribution of the four most common respiratory viruses among children hospitalized with acute respiratory infections from January 2001 to December 2011 in a subtropical area of China. The relationships between mean temperature and prevalence of respiratory syncytial virus (RSV; 1A), influenza A (IV-A; 1B), parainfluenza 3 (PIV-3; 1C) and adenovirus (ADV; 1D) are shown
Description of meteorological data
From 2001 to 2011, the monthly mean temperature was 16.8 ± 8.5 (mean ± standard deviation) °C, humidity was 67.3 ± 4.5 %, total rainfall was 86.2 ± 51.3 mm, total sunshine was 141.2 ± 50.7 h, and wind velocity was 1.8 ± 0.4 m/s. The monthly mean data for these meteorological variables over the study period are shown in Figure 2. With regard to the seasonal pattern, all meteorological factors were higher in summer and lower in winter (Table 3, P < 0.001 for all meteorological factors). However, statistical significance is shown only for mean temperature for the each season with pairwise comparisons (Table 3).
Bivariate relationship of meteorological factors with viral ARIs
Lower levels of these meteorological factors were correlated with increased prevalence of RSV infection, and higher levels were correlated with increased activities of PIV-3 and ADV (except for relative humidity). IV-A activity was correlated with low temperature, while IV-B activity was correlated with high wind velocity (Table 4).
Using partial correlations, we found that activities of RSV and PIV-3 were correlated with low and high environmental temperatures, respectively. IV-A activity was correlated with both low environmental temperature and high wind velocity, and IV-B activity was correlated with high wind velocity. ADV activity was correlated only with high rainfall (Table 5).
Multiple time series analysis
The results of time-series analysis using expert modeler for respiratory viruses are shown in Table 6. Environmental temperature had an impact on RSV, PIV-3 and ADV activity. RSV prevalence increased with low environmental temperature and PIV-3 and ADV prevalence increased with high environmental temperatures (Fig. 1).
The prevalence of RSV in hospitalized children was then predicted using the ARIMA model. Temperature was the only independent covariant significantly associated with the prevalence of RSV infection in the multiple time series analysis (β = −0.998, P = 0.002) (Fig. 3). The stationary R2 value was 0.612 for the ARIMA model automatically selected by the expert modeling procedure. No meteorological factors were included in the multiple time series analysis based on the ARIMA model for other respiratory viruses. The effect of temperature was significant at the lag periods of 2, 4, 5 and 7 months using the distributed lag model. Little effect was observed for the other lag periods (Fig. 4). Considerations were made for interpretational difficulty due to very long lag periods; we considered lag periods (delays in effect) of up to 3 months for the influence of temperature on the number of RSV cases. Eventually, notable significance (P = 0.0004) was found with lag periods of 2 months, with the same result when the analysis was done by ARIMA. For a 1 °C increase in the average monthly temperature, the number of RSV cases decreased by 11.25 % (95 % CI: 5.34 % to 16.79 %).
Prediction of the incidence of respiratory syncytial virus (RSV) infection on the basis of an autoregressive integrated moving average model (0,1,2), with mean temperature as the covariate. Good agreement was found between observed and predicted RSV incidence. LCL, lower confidence interval; UCL, upper confidence interval
Discussion
Infections caused by respiratory viruses have definite seasonality, but the reasons for seasonal variation are not clearly understood. A combination of immunological, epidemiological, and virological factors have been explained for this phenomenon [19]. Besides host and virus specific factors, meteorological factors are considered to contribute to this seasonality.
Our data demonstrated a complex interaction between common respiratory viruses and climatic factors including temperature, humidity, rainfall, sunshine and wind velocity. A complete understanding on how meteorological factors affect the transmission of the respiratory viruses would help with effectively predicting the spread of these viruses in humans. Although this may never be achieved, our study has increased the knowledge in this field.
In this study, environmental temperature was identified to be the most important meteorological factor associated with respiratory virus variations, especially for RSV, IV-A, and PIV-3. Monthly temperature was inversely correlated to RSV and IV-A activity, and positively correlated to PIV-3 activity, while IV-B activity correlated with both mean temperature and wind velocity. Total rainfall was positively correlated with ADV activity using partial correlations.
RSV was identified as to be the most common virus in hospitalized children with ARIs. Nevertheless, the seasonal prevalence of RSV infection varies between the tropical equatorial regions and the more temperate regions of the northern and southern hemispheres. In the geographical areas near the equator, such as Singapore, where the temperature is constantly high and rainfall is usually heavy, RSV activity was found throughout the year with a peak during March-August [12]. In Hawaii and Saudi Arabia, with temperate climate around a latitude 24° to 29°, RSV activity peaks during December-January, the wet winter season [20, 21]. In our study, we observed that RSV activity was consistent with that in Hawaii and Saudi Arabia, likely due to the similar climate, with a latitude of approximately 31°. Some temperature-based hypotheses center on human physiology in response to less sunlight and cold temperatures. A popular theory states reduced vitamin D production in winter in temperate climatic regions compromises the immune system, thus increasing host susceptibility to seasonal viruses [17]. People are more likely to spend time in a crowded, air-conditioned environment in winter, which favors the easy transmission of viruses from one individual to another as the environment favors survival of air-borne viruses [22]. Cold weather not only reduces the frequency of cilia beats that can help clear pathogens from uninfected individuals but also increases viral shedding from infected individuals [3, 23, 24]. In present study, RSV-associated hospitalizations were predictable, and the monthly number of RSV cases decreased by 11.25 % for every 1 °C increase in the average temperature with a lag effect.
For IV-A, there were no meteorological factors significantly associated in this study except for ambient temperature. The weekly incidence of influenza increased with lower environmental temperature and relative humidity in Japan [7]. A Hong Kong study reported increased incidence of IV-A with higher relative humidity [14]. Two studies conducted in a subtropical climate similar to ours showed differing results. One reason for this difference may be the relatively lower incidence of IV-A in Suzhou than in Japan and Hong Kong. The other reason may be that Suzhou is not a coastal but an inland city, in contrast to Hong Kong, and has a mean relative humidity of 68 % with a coefficient of variation of 8 %, which is much lower than that in Hong Kong. In this subtropical area, however, influenza B has a very low prevalence (0.6 %) throughout the year compared to influenza A (3.3 %), and the associations would not be obvious in this study.
In our study, PIV-3 activity demonstrated a clear annual epidemic cycle with a peak usually in late spring or summer, whereas PIV-1 and PIV-2 activity did not demonstrate any specific seasonality peaks. This finding is similar to a 22-year study conducted in England and Wales [25]. Hsieh et al. reported clusters of PIV-3 infections in Taiwan mainly in the spring and early summer over a 3-year study period [26]. PIV-3 activity in the present study had a positive association with monthly mean temperature. At the molecular level, temperature is known to influence the fusion of lipid-enveloped viruses such as PIV-3 with the cellular membrane, allowing cell entry and replication to occur. Viruses cannot effectively fuse with a cell and inject genetic material at low temperature [27, 28].
Our data on predicting respiratory virus activity could provide assistance in geographical areas where detection of respiratory virus activity may never be possible. The limitation of our study is that the data analysis alone may not serve as a conclusive interpretation, since any of the relationships with meteorological factors may be an indication of other social or environmental factors that also vary with the seasons. Furthermore, the question remains whether the incidence of virus in hospitalized children corresponds to that of the general community. It should also be noted that our study was based on a single center for data, which might have potential biases because of the age structure and vaccination coverage of the catchment population. In addition, the changing susceptibility of the population through physiological reactions to a certain climate could also be a factor, and transmission of respiratory viruses through physiological reactions and host behavior under certain climate conditions may also affect the interpretation of data [14, 19, 29–32].
Despite these limitations, further understanding of the impact of environmental factors on the prevalence of common respiratory viruses can be useful and important in predicting seasonality of respiratory virus infections. Different respiratory viruses show different correlations to certain meteorological factors. The ARIMA model predicted the prevalence of RSV infections on the basis of the influence of temperature and its own pattern.
References
Dowell SF (2001) Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis 7:369–374
Dowell SF, Ho MS (2004) Seasonality of infectious diseases and severe acute respiratory syndrome—what we don’t know can hurt us. Lancet Infect Dis 4:704–708
Lowen AC, Mubareka S, Steel J et al (2007) Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog 3:1470–1476
Chan PW, Chew FT, Tan TN et al (2002) Seasonal variation in respiratory syncytial virus chest infection in the tropics. Pediatr Pulm 34:47–51
Viegas M, Barrero PR, Maffey AF et al (2004) Respiratory viruses seasonality in children under five years of age in Buenos Aires, Argentina: a five-year analysis. J Infect 49:222–228
Lapeña S, Robles MB, Castañón L et al (2005) Climatic factors and lower respiratory tract infection due to respiratory syncytial virus in hospitalized infants in northern Spain. Eur J Epidemiol 20:271–276
Urashima M, Shindo N, Okabe N (2003) A seasonal model to simulate influenza oscillation in Tokyo. Jpn J Infect Dis 56:43–47
Wong CM, Yang L, Chan KP et al (2006) Influenza-associated hospitalization in a subtropical city. PLoS Med 3:e121
Alonso WJ, Viboud C, Simonsen L et al (2007) Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol 165:1434–1442
Omer SB, Sutanto A, Sarwo H et al (2008) Climatic, temporal, and geographic characteristics of respiratory syncytial virus disease in a tropical island population. Epidemiol Infect 136:1319–1327
Cox NJ, Subbarao K (2000) Global epidemiology of influenza: past and present. Annu Rev Med 51:407–421
Chew FT, Doraisingham S, Ling AE et al (1998) Seasonal trends of viral respiratory tract infections in the tropics. Epidemiol Infect 121:121–128
Chan PK, Mok HY, Lee TC et al (2009) Seasonal influenza activity in Hong Kong and its association with meteorological variations. J Med Virol 81:1797–1806
Tang JW, Lai FY, Wong F et al (2010) Incidence of common respiratory viral infections related to climate factors in hospitalized children in Hong Kong. Epidemiol Infect 138:226–235
Ji W, Wang Y, Chen Z et al (2009) Human metapneumovirus in children with acute respiratory tract infections in Suzhou, China 2005–2006. Scand J Infect Dis 41:735–744
Shafik CF, Mohareb EW, Youssef FG (2011) Comparison of direct fluorescence assay and real-time PCR as diagnostics for respiratory syncytial virus in young children. J Trop Med 2011:781919
Yusuf S, Piedimonte G, Auais A et al (2007) The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect 135:1077–1090
Onozuka D, Hashizume M, Hagihara A (2009) Impact of weather factors on Mycoplasma pneumoniae pneumonia. Thorax 64:507–511
Lofgren E, Fefferman NH, Naumov YN et al (2007) Influenza seasonality: underlying causes and modeling theories. J Virol 81:5429–5436
Reese PE, Marchette NJ (1991) Respiratory syncytial virus infection and prevalence of subgroups A and B in Hawaii. J Clin Microbiol 29:2614–2615
Jamjoom GA, al-Semrani AM, Board A et al (1993) Respiratory syncytial virus infection in young children hospitalized with respiratory illness in Riyadh. J Trop Pediatr 39:346–349
Tang JW (2009) The effect of environmental parameters on the survival of air-borne infectious agents. J R Soc Interface 6:737–746
Eccles R (2002) An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol 122:183–191
Pedersen M (1990) Ciliary activity and pollution. Lung 168:368–376
Laurichesse H, Dedman D, Watson JM et al (1999) Epidemiological features of parainfluenza virus infections: laboratory surveillance in England and Wales, 1975–1997. Eur J Epidemiol 15:475–484
Hsieh YJ, Chin H, Chiu NC et al (2010) Hospitalized pediatric parainfluenza virus infections in a medical center. J Microbiol Immunol Infect 43:360–365
Brunner J, Zugliani C, Mischler R (1991) Fusion activity of influenza virus PR8/34 correlates with a temperature-induced conformational change within the hemagglutinin ectodomain detected by photochemical labeling. Biochemistry 30:2432–2438
Yunus AS, Jackson TP, Crisafi K et al (2010) Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation. Virology 396:226–237
Dowling HF, Jackson GG, Spiesman IG et al (1958) Transmission of the common cold to volunteers under controlled conditions III. The effect of chilling of the subjects upon susceptibility. Am J Hyg 68:59–65
Eccles R (2002) Acute cooling of the body surface and the common cold. Rhinology 40:109–114
Yellon SM, Fagoaga OR, Nehlsen-Cannarella SL (1999) Influence of photoperiod on immune cell functions in the male Siberian hamster. Am J Physiol 276:97–102
Nelson MI, Holmes EC (2007) The evolution of epidemic influenza. Nat Rev Genet 8:196–205
Acknowledgement
This work was supported by Social Development, Science and Technology Projects of Jiangsu Province (W. Ji, Grant Number BE2012652) and Science and Technology Projects for the Youth of Suzhou (Z. R. Chen, Grant Number KJQND2011005).
Conflict of interest
The author(s) declare that they have no potential conflicts of interest with respect to the authorship and/or publication of this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yan Zhu is a co-first author.
Rights and permissions
About this article
Cite this article
Chen, Z., Zhu, Y., Wang, Y. et al. Association of meteorological factors with childhood viral acute respiratory infections in subtropical China: an analysis over 11 years. Arch Virol 159, 631–639 (2014). https://doi.org/10.1007/s00705-013-1863-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-013-1863-8