Abstract
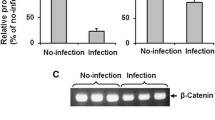
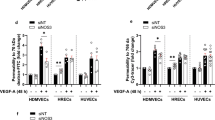
Hantaviruses infect human endothelial cells (ECs) and are known to cause vascular-permeability-based diseases, including hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). The αvβ3 integrins, which are highly expressed on the surface of ECs, serve as hantavirus receptors. Specifically, the β3 integrin and vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) form a functional complex and interact with each other. Signaling through this complex causes cytoskeletal reorganization, which is one of the most important mechanisms underlying hyperpermeability. In this study, we show that VEGF dramatically enhances Hantaan virus (HTNV)-directed permeability and increases the reorganization of the cytoskeleton and the disruption of junctional organizations in an EC monolayer at 3 days postinfection. HTNV infection reduced the effect of VEGF on adhesion, migration, and the upregulation of β3 expression, but the infection alone upregulated the expression of β3 and VEGFR2. These results indicate that in addition to its role in blocking β3 integrin activation as reported previously, HTNV blocks the function of the complex of VEGFR2 and β3 integrin, and the dysfunction of the complex may contribute to cytoskeletal reorganization in an HTNV-directed hyperpermeability response to VEGF.







Similar content being viewed by others
References
Bogatcheva NV, Garcia JG, Verin AD (2002) Role of tyrosine kinase signaling in endothelial cell barrier regulation. Vascul Pharmacol 39:201–212
Bogatcheva NV, Verin AD (2008) The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res 76:202–207
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146:1029–1039
Dvorak HF (2006) Discovery of vascular permeability factor (VPF). Exp Cell Res 312:522–526
Eliceiri BP (2001) Integrin and growth factor receptor crosstalk. Circ Res 89:1104–1110
Eum SY, Lee YW, Hennig B, Toborek M (2004) VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Exp Cell Res 296:231–244
Fuse C, Ishida Y, Hikita T, Asai T, Oku N (2007) Junctional adhesion molecule-C promotes metastatic potential of HT1080 human fibrosarcoma. J Biol Chem 282:8276–8283
Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER (1998) beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci USA 95:7074–7079
Gavrilovskaya IN, Brown EJ, Ginsberg MH, Mackow ER (1999) Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J Virol 73:3951–3959
Gavrilovskaya IN, Peresleni T, Geimonen E, Mackow ER (2002) Pathogenic hantaviruses selectively inhibit beta3 integrin directed endothelial cell migration. Arch Virol 147:1913–1931
Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER (2008) Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J Virol 82:5797–5806
Geiger B, Bershadsky A, Pankov R, Yamada KM (2001) Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Natl Rev Mol Cell Biol 2:793–805
Lee HW (1982) Hemorrhagic fever with renal syndrome (HFRS). Scand J Infect Dis 36:82–85
Larson RS, Brown DC, Ye C, Hjelle B (2005) Peptide antagonists that inhibit Sin Nombre virus and hantaan virus entry through the beta3-integrin receptor. J Virol 79:7319–7326
LeDuc JW, Childs CJ, Glass GE (1992) The Hantaviruses, etiologic agents of hemorrhagic fever with renal syndrome: a possible cause of hypertension and chronic renal disease in the United States. Annu Rev Public Health 13:79–98
Mackow ER, Gavrilovskaya IN (2001) Cellular receptors and hantavirus pathogenesis. Curr Top Microbiol Immunol 256:91–115
Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV (2006) Integrin signaling is critical for pathological angiogenesis. J Exp Med 203:2495–2507
Mou DL, Wang YP, Huang CX, Li GY, Pan L, Yang WS, Bai XF (2006) Cellular entry of Hantaan virus A9 strain: specific interactions with beta3 integrins and a novel 70kDa protein. Biochem Biophys Res Commun 339:611–617
Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM (2000) Interactions between cancer cells and the endothelium in metastasis. J Pathol 190:310–329
Palecek SP, Horwitz AF, Lauffenburger DA (1999) Kinetic model for integrin-mediated adhesion release during cell migration. Ann Biomed Eng 27:219–235
Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE (1995) Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell 6:1349–1365
Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F (1999) Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J 18:882–892
Stewart PL, Nemerow GR (2007) Cell integrins: commonly used receptors for diverse viral pathogens. Trends Microbiol 15:500–507
Sugimori T, Griffith DL, Arnaout MA (1997) Emerging paradigms of integrin ligand binding and activation. Kidney Int 51:1454–1462
Valbuena G, Walker DH (2006) The endothelium as a target for infections. Annu Rev Pathol 1:171–198
Wu MH (2005) Endothelial focal adhesions and barrier function. J Physiol 569:359–366
Yamada KM, Even-Ram S (2002) Integrin regulation of growth factor receptors. Nat Cell Biol 4:E75–E76
Zachary I, Gliki G (2001) Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res 49:568–581
Acknowledgments
This work was supported by the grant from National Basic Research Program of China (973 Program) (No. 2012CB518905) and National Natural Science Foundation of China (No. 30872215).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, W., Zhang, Y., Li, Y. et al. Dysregulation of the β3 integrin-VEGFR2 complex in Hantaan virus–directed hyperpermeability upon treatment with VEGF. Arch Virol 157, 1051–1061 (2012). https://doi.org/10.1007/s00705-012-1245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1245-7