Abstract
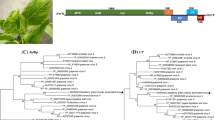
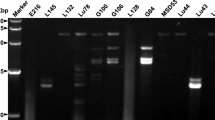
Two co-infecting novel vitiviruses from Actinidia chinensis were identified from mechanically inoculated Nicotiana occidentalis. Both virus genomes were sequenced and share 64% nucleotide identity. Their overall structure is typical of vitiviruses, with five open reading frames (ORFs) and a polyadenylated 3′ end. Open reading frame 4 (ORF4) encodes the coat protein, the most conserved gene of the vitiviruses, in which they share 75% amino acid identity, 61-68% with grapevine virus B, 55-59% with grapevine virus A, and 37-42% with grapevine virus E. Based on the molecular criteria for species demarcation in the family Betaflexiviridae, these are two novel viruses, tentatively named Actinidia virus A and Actinidia virus B.



Similar content being viewed by others
Reference
Adams MJ, Antoniw JF, Bar-Joseph M, Brunt AA, Candresse T, Foster GD, Martelli GP, Milne RG, Zavriev SK, Fauquet CM (2004) The new plant virus family Flexiviridae and assessment of molecular criteria for species demarcation. Arch Virol 149:1045–1060
Auger J, Perez I, Fullerton RA, Esterio M (2009) First report of Verticillium Wilt of Gold Kiwifruit, Actinidia chinensis Cv. Hort 16A, Caused by Verticillium albo-atrum in Chile. Plant Dis 93:553
Blouin AG, Greenwood DR, Chavan RR, Pearson MN, Clover GRG, MacDiarmid RM, Cohen D (2010) A generic method to identify plant viruses by high-resolution tandem mass spectrometry of their coat proteins. J Virol Methods 163:49–56
Boscia D, Savino V, Minafra A, Namba S, Elicio V, Castellano MA, Gonsalves D, Martelli GP (1993) Properties of a filamentous virus isolated from grapevines affected by corky bark. Arch Virol 130:109–120
Caciagli P, Lovisolo O (1987) Behaviour of kiwifruit plants (Actinidia deliciosa) in regard to virus infections. Adv Hortic Sci 1:11–14
Carstens EB (2010) Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009). Arch Virol 155:133–146
Chavan RR, Pearson MN, Cohen D (2009) Partial characterisation of a novel Tobamovirus infecting Actinidia chinensis and A. deliciosa (Actinidiaceae) from China. Eur J Plant Pathol 124:247–259
Chiba M, Reed JC, Prokhnevsky AI, Chapman EJ, Mawassi M, Koonin EV, Carrington JC, Dolja VV (2006) Diverse suppressors of RNA silencing enhance agroinfection by a viral replicon. Virology 346:7–14
Clover GRG, Pearson MN, Elliott DR, Tang Z, Smales TE, Alexander BJR (2003) Characterization of a strain of Apple stem grooving virus in Actinidia chinensis from China. Plant Pathol 52:371–378
Coetzee B, Maree HJ, Stephan D, Freeborough M-J, Burger JT (2010) The first complete nucleotide sequence of a grapevine virus E variant. Arch Virol 155:1357–1360
Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A (2011) Geneious v5.4. http://www.geneious.com/
du Preez J, Stephan D, Mawassi M, Burger JT (2011) The grapevine-infecting vitiviruses, with particular reference to grapevine virus A. Arch Virol 156:1495–1503
Elena K (2009) Occurrence of Phomopsis sp. on kiwi plantations in Northern Greece. Hellenic Plant Prot J 2:67–69
Everett K, Taylor R, Romberg M, Rees-George J, Fullerton R, Vanneste J, Manning M (2011) First report of Pseudomonas syringae pv. actinidiae causing kiwifruit canker in New Zealand. Australasian Plant Dis Notes 6:67–71
Fauquet C, Mayo M, Maniloff J, Desselberger U, Ball L (2005) Virus taxonomy: eighth report of the international committee on taxonomy of viruses. Elsevier Academic Press, San Diego
Ferguson AR, Seal AG (2008) Kiwifruit. In: Hancock JF (ed) Temperate fruit crop breeding. Springer, The Netherlands, pp 235–264
Fresh Facts (2009) Fresh Facts: New Zealand horticulture. The New Zealand Institute for Plant and Food Research Limited. http://www.plantandfood.co.nz/file/freshfacts-brochure-2009.pdf
Galiakparov N, Tanne E, Mawassi M, Gafny R, Sela I (2003) ORF 5 of grapevine virus A encodes a nucleic acid-binding protein and affects pathogenesis. Virus Genes 27:257–262
Gorbalenya AE, Koonin EV (1993) Helicases—amino-acid-sequence comparisons and structure-function-relationships. Curr Opin Struct Biol 3:419–429
Harrell L, Melcher U, Atkins JF (2002) Predominance of six different hexanucleotide recoding signals 3′ of read-through stop codons. Nucleic Acids Res 30:2011–2017
Hommay G, Komar V, Lemaire O, Herrbach E (2008) Grapevine virus A transmission by larvae of Parthenolecanium corni. Eur J Plant Pathol 121:185–188
Horner IJ (1992) Epidemiology of armillaria root-rot of kiwifruit. Second international symposium on kiwifruit. Acta Horticulturae 297:573–578
Koh YJ, Nou IS (2002) DNA markers for identification of Pseudomonas syringae pv. actinidiae. Mol Cells 13:309–314
Koonin EV, Dolja VV (1993) Evolution and taxonomy of positive-strand RNA viruses—implications of comparative-analysis of amino-acid-sequences. Crit Rev Biochem Mol Biol 28:375–430
La Notte P, Buzkan N, Choueiri E, Minafra A, Martelli GP (1997) Acquisition and transmission of grapevine virus A by the mealybug Pseudococcus longispinus. J Plant Pathol 78:79–85
Martelli GP, Minafra A, Saldarelli P (1997) Vitivirus, a new genus of plant viruses. Arch Virol 142:1929–1932
Martelli GP, Adams MJ, Kreuze JF, Dolja VV (2007) Family Flexiviridae: a case study in virion and genome plasticity. Annu Rev Phytopathol 45:73–100
Nakaune R, Toda S, Mochizuki M, Nakano M (2008) Identification and characterization of a new vitivirus from grapevine. Arch Virol 153:1827–1832
Nitta H, Ogasawara S (1997) A new, graft-transmitted, disease of kiwifruit. Third international symposium on kiwifruit. Acta Horticulturae 444:739–743
Pearson MN, Cohen D, Chavan R, Blouin A, Cowell SJ (2009) Molecular characterisation of viruses from kiwifruit. XXIst international conference on virus other graft transmissible diseases of fruit crops. Julius-Kuhn-Archiv 427:87–91. http://www.jki.bund.de/fileadmin/dam_uploads/_veroeff/JKI_Archiv/JKI_Archiv_427.pdf
Prodi A, Sandalo S, Tonti S, Nipoti P, Pisi A (2008) Phialophora-like fungi associated with kiwifruit elephantiasis. J Plant Pathol 90:487–494
Rees-George J, Vanneste JL, Cornish DA, Pushparajah IPS, Yu J, Templeton MD, Everett KR (2010) Detection of Pseudomonas syringae pv. actinidiae using polymerase chain reaction (PCR) primers based on the 16S–23S rDNA intertranscribed spacer region and comparison with PCR primers based on other gene regions. Plant Pathol 59:453–464
Roossinck MJ (2011) The good viruses: viral mutualistic symbioses. Nat Rev Microbiol 9:99–108
Tu X, Shen Z, Gao Z, Kang Z, Huang L, Tu X, Shen Z, Gao ZP, Kang ZS, Huang LL (2011) Investigation of kiwifruit bacterial canker disease (Pseudomonas syringae pv. Actinidiae Takikawa) in Guanzhong area of Shanxi Province and its biological control. Plant Dis Pests 2:17–20
Tzanetakis IE, Postman JD, Martin RR (2007) Identification, detection and transmission of a new vitivirus from Mentha. Arch Virol 152:2027–2033
Vanneste JL, Poliakoff F, Audusseau C, Cornish DA, Paillard S, Rivoal C, Yu J (2011) First Report of Pseudomonas syringae pv. actinidiae, the causal agent of bacterial canker of Kiwifruit in France. Plant Dis 95:1311–1312
Zhou ZS, Dell’Orco M, Saldarelli P, Turturo C, Minafra A, Martelli GP (2006) Identification of an RNA-silencing suppressor in the genome of Grapevine virus A. J Gen Virol 87:2387–2395
Acknowledgements
This research was supported partially by the Foundation for Research, Science and Technology, New Zealand (C06X0710), and capability funding from The New Zealand Institute for Plant & Food Research Ltd. The authors would like to thank Donald Hunter for his valuable suggestions on this manuscript and Gardette Valmonte for her help with phylogenetic analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blouin, A.G., Chavan, R.R., Pearson, M.N. et al. Detection and characterisation of two novel vitiviruses infecting Actinidia . Arch Virol 157, 713–722 (2012). https://doi.org/10.1007/s00705-011-1219-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-011-1219-1