Abstract
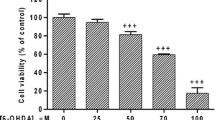
Over the years, evidence has accumulated on a possible contributive role of the cytosolic quinone reductase NQO2 in models of dopamine neuron degeneration induced by parkinsonian toxin, but most of the data have been obtained in vitro. For this reason, we asked the question whether NQO2 is involved in the in vivo toxicity of MPTP, a neurotoxin classically used to model Parkinson disease-induced neurodegeneration. First, we show that NQO2 is expressed in mouse substantia nigra dopaminergic cell bodies and in human dopaminergic SH-SY5Y cells as well. A highly specific NQO2 inhibitor, S29434, was able to reduce MPTP-induced cell death in a co-culture system of SH-SY5Y cells with astrocytoma U373 cells but was inactive in SH-SY5Y monocultures. We found that S29434 only marginally prevents substantia nigra tyrosine hydroxylase+ cell loss after MPTP intoxication in vivo. The compound produced a slight increase of dopaminergic cell survival at day 7 and 21 following MPTP treatment, especially with 1.5 and 3 mg/kg dosage regimen. The rescue effect did not reach statistical significance (except for one experiment at day 7) and tended to decrease with the 4.5 mg/kg dose, at the latest time point. Despite the lack of robust protective activity of the inhibitor of NQO2 in the mouse MPTP model, we cannot rule out a possible role of the enzyme in parkinsonian degeneration, particularly because it is substantially expressed in dopaminergic neurons.





Similar content being viewed by others
Data availability
Data are available upon request.
Abbreviations
- DN:
-
Dopaminergic neurons
- DAB:
-
Diaminobenzidine
- DPI:
-
Days post-injection
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+ :
-
1-Methyl-4-phenylpyridinium
- NQO2:
-
Quinone reductase 2
- OD:
-
Optical density
- PBS:
-
Phosphate buffer saline
- PD:
-
Parkinson disease
- ROS:
-
Reactive oxygen species
- SEM:
-
Standard error of the mean
- SN:
-
Substantia nigra
- SNpc:
-
Substantia nigra pars compacta
- TH:
-
Tyrosine hydroxylase
References
Asanuma M, Miyazaki I, Ogawa N (2003) Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res 5(3):165–176
Benoit C-E, Bastianetto S, Brouillette J, Tse Y, Boutin JA, Delagrange P, Wong T, Sarret P, Quirion R (2010) Loss of quinone reductase 2 function selectively facilitates learning behaviors. J Neurosci 30(38):12690–12700
Boutin JA, Bouillaud F, Janda E, Gacsalyi I, Guillaumet G, Hirsch EC, Kane DA, Nepveu F, Reybier K, Dupuis P, Bertrand M, Chhour M, Le Diguarher T, Antoine M, Brebner K, Da Costa H, Ducrot P, Giganti A, Goswami V, Guedouari H, Michel PP, Patel A, Paysant J, Stojko J, Viaud-Massuard M-C, Ferry G (2019) S29434, a quinone reductase 2 inhibitor: main biochemical and cellular characterization. Mol Pharmacol 95(3):269–285
Brouillette J, Quirion R (2008) Transthyretin: a key gene involved in the maintenance of memory capacities during aging. Neurobiol Aging 29(11):1721–1732
Cassagnes L-E, Perio P, Ferry G, Moulharat N, Antoine M, Gayon R, Boutin JA, Nepveu F, Reybier K (2015) In cellulo monitoring of quinone reductase activity and reactive oxygen species production during the redox cycling of 1,2 and 1,4 quinones. Free Radic Biol Med 89:126–134
Cassagnes L-E, Chhour M, Pério P, Sudor J, Gayon R, Ferry G, Boutin JA, Nepveu F, Reybier K (2018) Oxidative stress and neurodegeneration: the possible contribution of quinone reductase 2. Free Radic Biol Med 120:56–61
Chang K-H, Chen C-M (2020) The role of oxidative stress in Parkinson’s disease. Antioxidants (basel) 9(7):597
Corasaniti MT, Bagetta G, Rodinò P, Gratteri S, Nisticò G (1992) Neurotoxic effects induced by intracerebral and systemic injection of paraquat in rats. Hum Exp Toxicol 11(6):535–539
Corasaniti MT, Strongoli MC, Rotiroti D, Bagetta G, Nisticò G (1998) Paraquat: a useful tool for the in vivo study of mechanisms of neuronal cell death. Pharmacol Toxicol 83(1):1–7
Cristóvão AC, Choi D-H, Baltazar G, Beal MF, Kim Y-S (2009) The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal 11(9):2105–2118
Dehay B, Martinez-Vicente M, Caldwell GA, Caldwell KA, Yue Z, Cookson MR, Klein C, Vila M, Bezard E (2013) Lysosomal impairment in Parkinson’s disease. Mov Disord 28(6):725–732
Drechsel DA, Patel M (2008) Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Rad Biol Med 44(11):1873–1886
Fu Y, Buryanovskyy L, Zhang Z (2008) Quinone reductase 2 is a catechol quinone reductase. J Biol Chem 283(35):23829–23835
Gonzalez-Sepulveda M, Compte J, Cuadros T, Nicolau A, Guillard-Sirieix C, Peñuelas N, Lorente-Picon M, Parent A, Romero-Giménez J, Cladera-Sastre JM, Laguna A, Vila M (2023) In vivo reduction of age-dependent neuromelanin accumulation mitigates features of Parkinson’s disease. Brain 146(3):1040–1052
Gould NL, Sharma V, Hleihil M, Kolatt Chandran S, David O, Edry E, Rosenblum K (2020) Dopamine-dependent QR2 pathway activation in CA1 interneurons enhances novel memory formation. J Neurosci 40(45):8698–8714
Gould NL, Scherer GR, Carvalho S, Shurrush K, Kayyal H, Edry E, Elkobi A, David O, Foqara M, Thakar D, Pavesi T, Sharma V, Walker M, Maitland M, Dym O, Albeck S, Peleg Y, Germain N, Babaev I, Sharir H, Lalzar M, Shklyar B, Hazut N, Khamaisy M, Lévesque M, Lajoie G, Avoli M, Amitai G, Lefker B, Subramanyam C, Shilton B, Barr H, Rosenblum K (2023) Specific quinone reductase 2 inhibitors reduce metabolic burden and reverse Alzheimer’s disease phenotype in mice. J Clin Invest 133:e162120
Han X, Zhao S, Song H, Xu T, Fang Q, Hu G, Sun L (2021) Kaempferol alleviates LD-mitochondrial damage by promoting autophagy: Implications in Parkinson’s disease. Redox Biol 41:101911
Harada S, Fujii C, Hayashi A, Ohkoshi N (2001) An association between idiopathic Parkinson’s disease and polymorphisms of phase II detoxification enzymes: glutathione S-transferase M1 and quinone oxidoreductase 1 and 2. Biochem Biophys Res Commun 288(4):887–892
Hashimoto T, Nakai M (2011) Increased hippocampal quinone reductase 2 in Alzheimer’s disease. Neurosci Lett 502(1):10–12
Hirsch EC (1992) Why are nigral catecholaminergic neurons more vulnerable than other cells in Parkinson’s disease? Ann Neurol 32(Suppl):S88–S93
Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334(6180):345–348
Hirsch EC, Höglinger G, Rousselet E, Breidert T, Parain K, Feger J, Ruberg M, Prigent A, Cohen-Salmon C, Launay JM (2003) Animal models of Parkinson’s disease in rodents induced by toxins: an update. J Neural Transm Suppl 65:89–100
Huang M, Bargues-Carot A, Riaz Z, Wickham H, Zenitsky G, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG (2022) Impact of environmental risk factors on mitochondrial dysfunction, neuroinflammation, protein misfolding, and oxidative stress in the etiopathogenesis of Parkinson’s Disease. Int J Mol Sci 23(18):10808
Jaiswal AK (1994) Human NAD(P)H:quinone oxidoreductase2. Gene structure, activity, and tissue-specific expression. J Biol Chem 269(20):14502–14508
Janda E, Isidoro C, Carresi C, Mollace V (2012) Defective autophagy in Parkinson’s disease: role of oxidative stress. Mol Neurobiol 46(3):639–661
Janda E, Parafati M, Aprigliano S, Carresi C, Visalli V, Sacco I, Ventrice D, Mega T, Vadalá N, Rinaldi S, Musolino V, Palma E, Gratteri S, Rotiroti D, Mollace V (2013) The antidote effect of quinone oxidoreductase 2 inhibitor against paraquat-induced toxicity in vitro and in vivo. Br J Pharmacol 168(1):46–59
Janda E, Lascala A, Carresi C, Parafati M, Aprigliano S, Russo V, Savoia C, Ziviani E, Musolino V, Morani F, Isidoro C, Mollace V (2015) Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: Implications for neuroprotection. Autophagy 11(7):1063–1080
Janda E, Nepveu F, Calamini B, Ferry G, Boutin JA (2020) Molecular pharmacology of NRH: quinone oxidoreductase 2: a detoxifying enzyme acting as an undercover toxifying enzyme. Mol Pharmacol 98(5):620–633
Janda E, Martino C, Riillo C, Parafati M, Lascala A, Mollace V, Boutin JA (2021) Apigenin and luteolin regulate autophagy by targeting NRH-quinone oxidoreductase 2 in liver cells. Antioxidants (basel) 10(5):776
Janda E, Parafati M, Martino C, William JNG, Reybier K, Mollace V, Boutin JA (2023) Autophagy and neuroprotection in astrocytes exposed to 6-hydroxydopamine is negatively regulated by NQO2: a potential novel target in Parkinson’s disease. Sci Reports. https://doi.org/10.21203/rs.3.rs-2510273/v1
Johannessen JN, Adams JD, Schuller HM, Bacon JP, Markey SP (1986) 1-methyl-4-phenylpyridine (MPP+) induces oxidative stress in the rodent. Life Sci 38(8):743–749
Kartik S, Pal R, Chaudhary MJ, Nath R, Kumar M, Binwal M, Bawankule DU (2023) Neuroprotective role of chloroquine via modulation of autophagy and neuroinflammation in MPTP-induced Parkinson’s disease. Inflammopharmacology 31(2):927–941
Kastner A, Hirsch EC, Herrero MT, Javoy-Agid F, Agid Y (1993) Immunocytochemical quantification of tyrosine hydroxylase at a cellular level in the mesencephalon of control subjects and patients with Parkinson’s and Alzheimer’s disease. J Neurochem 61(3):1024–1034
Kupsch A, Schmidt W, Gizatullina Z, Debska-Vielhaber G, Voges J, Striggow F, Panther P, Schwegler H, Heinze H-J, Vielhaber S, Gellerich FN (2014) 6-Hydroxydopamine impairs mitochondrial function in the rat model of Parkinson’s disease: respirometric, histological, and behavioral analyses. J Neural Transm (vienna) 121(10):1245–1257
Langston JW (2017) The MPTP story. J Parkinson’s Dis 7(s1):S11–S19
Langston JW, Irwin I, Langston EB, Forno LS (1984) Pargyline prevents MPTP-induced parkinsonism in primates. Science 225(4669):1480–1482
Lim C-Y, Zoncu R (2016) The lysosome as a command-and-control center for cellular metabolism. J Cell Biol 214(6):653–664
Liu Z, Zhuang W, Cai M, Lv E, Wang Y, Wu Z, Wang H, Fu W (2023) Kaemperfol protects dopaminergic neurons by promoting mTOR-mediated autophagy in Parkinson’s disease models. Neurochem Res 48(5):1395–1411
Lu H, Chen J, Huang H, Zhou M, Zhu Q, Yao SQ, Chai Z, Hu Y (2017) Iron modulates the activity of monoamine oxidase B in SH-SY5Y cells. Biometals 30(4):599–607
Mailliet F, Ferry G, Vella F, Thiam K, Delagrange P, Boutin JA (2004) Organs from mice deleted for NRH:quinone oxidoreductase 2 are deprived of the melatonin binding site MT3. FEBS Lett 578(1–2):116–120
Nolan KA, Humphries MP, Barnes J, Doncaster JR, Caraher MC, Tirelli N, Bryce RA, Whitehead RC, Stratford IJ (2010) Triazoloacridin-6-ones as novel inhibitors of the quinone oxidoreductases NQO1 and NQO2. Bioorg Med Chem 18(2):696–706
Nosjean O, Nicolas J-P, Klupsch F, Delagrange P, Canet E, Boutin JA (2001) Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2 tissue distribution of MT3/QR2. Biochem Pharmacol 61(11):1369–1379
Pantic I, Cumic J, Skodric SR, Dugalic S, Brodski C (2021) Oxidopamine and oxidative stress: recent advances in experimental physiology and pharmacology. Chem Biol Interact 336:109380
Ran L-Y, Xiang J, Zeng X-X, He W-W, Dong Y-T, Yu W-F, Qi X-L, Xiao Y, Cao K, Zou J, Guan Z-Z (2023) The influence of NQO2 on the dysfunctional autophagy and oxidative stress induced in the hippocampus of rats and in SH-SY5Y cells by fluoride. CNS Neurosci Ther 29(4):1129–1141
Rappaport AN, Jacob E, Sharma V, Inberg S, Elkobi A, Ounallah-Saad H, Pasmanik-Chor M, Edry E, Rosenblum K (2015) Expression of quinone reductase-2 in the cortex is a muscarinic acetylcholine receptor-dependent memory consolidation constraint. J Neurosci 35(47):15568–15581
Reybier K, Perio P, Ferry G, Bouajila J, Delagrange P, Boutin JA, Nepveu F (2011) Insights into the redox cycle of human quinone reductase 2. Free Radic Res 45(10):1184–1195
Sollner S, Macheroux P (2009) New roles of flavoproteins in molecular cell biology: an unexpected role for quinone reductases as regulators of proteasomal degradation. FEBS J 276(16):4313–4324
Testa B, Krämer SD (2007) The biochemistry of drug metabolism—an introduction: Part 2. Redox reactions and their enzymes. Chem Biodivers 4(3):257–405
Vakhitova YV, Kuzmina US, Voronin MV, Zainullina LF, Seredenin SB (2019) Effect of fabomotizole on brain gene expression in MR rats in the open field test. Dokl Biochem Biophys 488(1):313–315
Vella F, Ferry G, Delagrange P, Boutin JA (2005) NRH:quinone reductase 2: an enzyme of surprises and mysteries. Biochem Pharmacol 71(1–2):1–12
Voronin MV, Kadnikov IA, Zainullina LF, Logvinov IO, Verbovaya ER, Antipova TA, Vakhitova YV, Seredenin SB (2021) Neuroprotective properties of quinone reductase 2 inhibitor M-11, a 2-mercaptobenzimidazole derivative. Int J Mol Sci 22(23):13061
Yang J-H, Kondratyuk TP, Jermihov KC, Marler LE, Qiu X, Choi Y, Cao H, Yu R, Sturdy M, Huang R, Liu Y, Wang L-Q, Mesecar AD, van Breemen RB, Pezzuto JM, Fong HHS, Chen Y-G, Zhang H-J (2011) Bioactive compounds from the fern Lepisorus contortus. J Nat Prod 74(2):129–136
Zhao Q, Yang XL, Holtzclaw WD, Talalay P (1997) Unexpected genetic and structural relationships of a long-forgotten flavoenzyme to NAD(P)H:quinone reductase (DT-diaphorase). Proc Natl Acad Sci U S A 94(5):1669–1674
Acknowledgements
This work was realized with the equipment and services from the Histomics and ICMice platform at ICM. The authors would like to thank Nouhad Samb and Laetitia Da Costa for their technical assistance. This work was sponsored by InnovAction internal program of the Laboratoires Servier. JAB and GF wish to thank Dr. Emmanuel Canet for his support and visions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vallucci, M., Boutin, J.A., Janda, E. et al. The specific NQO2 inhibitor, S29434, only marginally improves the survival of dopamine neurons in MPTP-intoxicated mice. J Neural Transm 131, 1–11 (2024). https://doi.org/10.1007/s00702-023-02709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-023-02709-3