Abstract
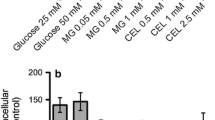
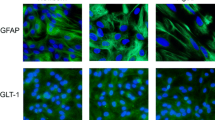
Advanced glycation endproducts (AGEs) arise from the reaction of sugars with side chains and the N-terminus of proteins and are thought to be involved in the pathogenesis of several diseases by inducing oxidative stress, inflammation and cell death presumably mediated through activation of the receptor of AGE (RAGE). To address the question whether the cell damaging effect of AGE depends on the degree of its protein glycation, differential modified AGEs derived from incubating human serum albumin with increasing concentrations of methyl glyoxal were tested on cell viability, reactive oxygen species (ROS) formation, intracellular ATP levels, and activation of caspases 3/7 in two human glial cell lines, which were used as a model for human glia cells. All AGEs tested, regardless of their degree of modification, were found to induce ROS formation in both microglial (CHME-5) and astroglial cells (U373 MG), while only highly modified AGEs were able to decrease the cell viability and to induce apoptosis. This indicates that apoptotic events may be involved in the change of physiological parameters.







Similar content being viewed by others
Abbreviations
- A:
-
Absorbance
- AD:
-
Alzheimer’s disease
- AGEs:
-
Advanced glycation endproducts
- BCA:
-
Bicinchoninic acid
- BSA:
-
Bovine serum albumin
- CTB:
-
CellTiter Blue
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FCS:
-
Fetal calf serum
- HBSS:
-
Hank’s balanced salt solution
- HSA:
-
Human serum albumin
- H2DCF-DA:
-
Dichlorodihydrofluorescein diacetate
- MG:
-
Methyl glyoxal
- PB:
-
Phosphate buffer
- PBS:
-
Phosphate buffered saline
- RAGE:
-
Receptor for AGEs
- ROS:
-
Reactive oxygen species
- RT:
-
Room temperature
- TFA:
-
Trifluoracetoacid
- w:
-
Weeks
References
Alikhani M, MacLellan C, Raptis M, Vora S, Trackman PC, Graves DT (2006) Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases and FOX01 transcription factor. Am J Physiol Cell Physiol, 27 September 27 (Epub)
Alikhani M, Alikhani Z, Boyd C, MacLellan C, Raptis M, Liu R, Pischon N, Trackman PC, Gerstenfeld L, Graves DT (2007) Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathway. Bone 40:345–353
de Arriba SG, Loske C, Meiners I, Fleischer G, Lobisch M, Wessel K, Tritschler H, Schinzel R, Münch G (2003) Advanced glycation endproducts induce changes in glucose concumption, lactate production, and ATP-levels in SH-SY5Y neuroblastoma cells by a redox-sensitive mechanism. J Cereb Blood Flow Metab 23:1307–1313
Basta G, Schmidt AM, De Caterina R (2004) Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetis. Cardiovasc Res 63:582–592
Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R (2005) At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol 25:1401–1407
Bigl K, Schmitt A, Meiners I, Münch G, Arendt T (2007) Comparison of results of the CellTiter Blue, the tetrazolium (3-[4,5-dimethylthioazol-2-yl]-2,5-diphenyl tetrazolium bromide), and the lactatedehydrogenase assay applied in brain cells after exposure to advanced glycation endproducts. Toxicol In Vitro, 21(5):962–971 (Epub 2007, 20 February)
Bouma B, Kroon-Batenburg LM, Wu YP, Brunjes B, Posthuma G, Kranenburg O, de Groot PG, Voest EE, Gebbink MF (2003) Glycation induces formation of amyloid cross-beta structure in albumin. J Biol Chem 278(43):41810–41819
Chen Bh, Jiang Dy, Tang Ls (2006) Advanced glycation endproducts induce apoptosis involving the singaling pathways of oxidative stress in bovine retinal pericytes. Life Sci 79:1040–1048
Dickson DW, Simicropi S, Yen SH, Ko LW, Mattiace LA, Bucala R, Vlassara H (1996) Glycation and microglial reaction in lesions of Alzheimer’s disease. Neurobiol Aging 17:733–743
Dukic-Stefankovic S, Gasic-Milenkovic J, Deuther-Conrad W, Münch G (2003) Signal transductions pathways in mouse microgial N-11 cells activated by advanced glycationd endproducts (AGEs). J Neurochem 87:44–55
Fan X, Subramaniam R, Weiss MF, Monnier VM (2003) Methylglyoxal-bovine serum albumin stimulates tumor necrosis factor alpha secretion in RAW 264.7 cells through activation of mitogen-activating protein kinase, nuclear factor κB and intracellular reactive oxygen species formation. Arch Biochem Biophys 409:274–286
Fukami K, Ueda S, Yamagishi S, Kato S, Inagaki Y, Takeuchi M, Motomiya Y, Bucala R, Iida S, Tamaki K, Imaizumi T, Cooper ME, Okuda S (2004) AGEs activate mesangial TGF-beta-Smad signalling via an angiotensin II type I receptor interaction. Kidney Int 66:2137–2147
Gasic-Milenkovic J, Dukic-Stefanovic S, Deuther-Conrad W, Gärtner U, Münch G (2003) ß-Amyloid peptide potentiates inflammatory responses induced by lipopolysaccharide, interferon-γ and “advanced glycation endproducts” in a murine microglia cell line. Eur J Neurosci 17:813–821
Gaunitz F, Heise K (2003) HTS compatible assay for antioxidative agents using primary cultured hepatocytes. Assay Drug Dev Technol 1(3):469–477
Janabi N, Peudenier S, Heron B, Ng KH, Tardieu M (1995) Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV 40 large T antigen. Neurosci Lett 195(2):105–108
Kitamura Y, Nomura Y (2003) Stress proteins and glial functions: possible therapeutic targets for neurodegenerative disorders. Pharmacol Ther 97:35–53
Kuhla B, Loske C, de Arriba SG, Schinzel R, Huber J, Münch G (2004) Differential effects of “Advanced Glycation Endproducts” and ß-amyloid peptide on glucose utilitzation and ATP levels in the neuronal cell line SH-SY5Y. J Neural Tansmission 111:427–439
Kuhla B, Lüth HJ, Haferburg D, Boeck K, Arendt T, Münch G (2005) Methylglyoxal, Glyoxal and their detoxification in Alzheimer’s disease. Ann N Y Acad Sci 1043:211–216
Li JJ, Dickson D, Hof PR, Vlassara H (1998) Receptors for advanced glycosylation endproducts in human brain: role in brain homeostasis. Mol Med 4(1):46–60
Loske C, Neumann A, Cunningham AM, Nichol K, Schinzel R, Riederer P, Münch G (1998) Cytotoxicity of advanced glycation endproducts is mediated by oxidative stress. J Neural Transmission 105:1005–1015
Mamputu C, Renier G (2004) Signalling pathways involved in retinal endothelial cell proliferation induced by advanced glycation end products: inhibitory effect of gliclazide. Diabetes Obes Metab 6:95–103
Münch G, Taneli Y, Schraven E, Schindler U, Schinzel R, Palm D, Riederer P (1994) The cognition-enhancing drug tenilsetam is an inhibitor of protein crosslinking by advanced glycosylation. J Neural Transmission Park Dis Dement Sect 8:193–208
Münch G, Schinzel R, Loske C, Wong A, Durany N, Li JJ, Vlassara H, Smith MA, Perry G, Riederer P (1998) Alzheimer’s disease—synergistic effects of glucose deficite, oxidative stress and advanced glycation endproducts. J Neural Transmission 105(4–5):439–461
Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA (2002) Tumor necrosis factor inhibits neurite outgrowth and branching of hippopampal neurons by a Rho-dependent mechanism. J Neurosci 22(3):854–862
Okano Y, Masaki H, Sakurai H (2002) Dysfunction of dermal fibroblasts induced by advanced glycation end-products (AGEs) and the contribution of a non-specific interaction with cell membrane and AGEs. J Dermatol Sci 29:171–180
Sadek HA, Humphries KM, Szweda PA, Szweda LI (2002) Selective inactivation of redox-sensitive mitochondrial enzymes during cardiac reperfusion. Arch Biochem Biophys 406:222–228
Sasaki N, Fukatsu R, Tsuzuki K, Hayashi Y, Ysoshida T, Fujiii N, Kokike T, Wakayma I, Yanagihara R, Garruto R, Amano N, Makita Z (1998) Advanced glycation endproducts in Alzheimer’s disease and other neurodegenerative diseases. Am J Pathol 153:1149–1155
Scheubel RJ, Kahrstedt S, Weber H, Holtz J, Friedrich I, Borgermann J, Silber RE, Simm A (2006) Depression of progenitor cell function by advanced glycation endproducts (AGEs): potential relevance for impaired angiogenesis in advanced age and diabetes. Exp Gerontol 41(5):540–580
Schmitt A, Meiners I, Schmitt J, Nöller J, Ihling C, Münch G, Sinz A, Nieber K (2005a) Two analytical methods to study the interaction of AGEs with cell surface proteins. J Biochem Biophys Methods 65:121–136
Schmitt A, Gasic-Milenkovic J, Schmitt J (2005b) Characterisation of advanced glycation end products: Mass changes in correlation to side chain modifications. Anal Biochem 346:101–106
Schmitt A, Schmitt J, Münch G, Gasic-Milenkovic J (2005c) Characterisation of advanced glycation end products for biochemical studies: side chain modifications and fluorescence characteristics. Anal Biochem 338:201–215
Schmitt A, Bigl K, Meiners I, Schmitt J (2006) Induction of reactive oxygen species and cell survival in the presence of advanced glycation end products and similar structures. Biochimica et Biophysica Acta 1763:927–936
Schmitt A, Noller J, Schmitt J (2007) The binding of advanced glycation end products to cell surfaces can be measured using bead-reconstituted cellular membrane proteins. Biochim Biophys Acta Mar 30
Shapira R, Austin GE, Mirra SS (1988) Neuritic plaque amyloid in Alzheimer's disease is highly racemized. J Neurochem 50(1):69–74
Shuvaev VV, Laffont I, Serot JM, Fujii J, Taniguchi N, Siest G (2001) Increased protein glycation in cerebrospinal fluid of Alzheimer’s disease. Neurobiol Aging 22:397–402
Subramaniam R, Fan XJ, Scivittaro V, Yang J, Ha CE, Petersen CE, Surewicz WK, Bhagavan NV, Weiss MF, Monnier VM (2002) Cellular oxidant stress and advanced glycation end products of albumin: caveats of the dichlorofluorescein assay. Arch Biochem Biophys 400:15–25
Thornalley PJ (1998) Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand) 44:1013–1023
Thornalley PJ (2002) methods for studying the binding of advanced glycated proteins to receptors for advanced glycationendproducts (AGE-receptors). In: Armstrong D (ed) Methods in molecular biology, oxidants and antioxidants: ultrastructure and molecular biology protocols, vol 196. Humana Press Inc, Totowa
Uchida Y, Ohba K, Yoshioka T, Irie K, Muraki T, Maru Y (2004) Cellular carbonyl stress enhances the expression of plasminogen activator inhibitor-1 in rat white adipocytes via reactive oxygen species-dependent pathway. J Biol Chem 279:4075–4083
Ulrich P, Cerami A (2001) Protein glycation, diabetis and aging. Recent Prog Horm Res 56:1–21
Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci USA 91(11):4766–4770
Wendt T, Tanji N, Guo J, Hudson BI, Bierhaus A, Ramasamy R, Arnold B, Nawroth PP, Yan SF, D’Agati V, Schmidt AM (2003) Glucose, glycation, and RAGE: implications for amplification of cellular dysfunction in diabetic nephropathy. J Am Soc Nephrol 14(5):1383–1395 (review)
Westwood ME, Thornalley PJ (1995) Molecular characteristics of methylglyoxal-modified bovine and human serum albumins. Comparison with glucose-derived advanced glycation endproduct-modified serum albumins. J Protein Chem 14:359–372
Wong A, Lüth HJ, Deuther-Conrad W, Dukic-Stefankovic S, Gasic-Milenkovic J, Arendt T, Münch G (2001) Advanced glycation endproducts co-localise with inducible nitric oxide synthase in Alzheimer’s disease. Brain Res 920:32–40
Wong A, Dukic-Stefankovic A, Gasic-Milenkovic J, Schinzel R, Wiesinger H, Riederer R, Münch G (2001) Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur J Neurosci 14:1961–1967
Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D (1994) Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem 269(13), pp 9889–9897 (issue of April 1)
Yan SF, Ramasamy R, Naka Y, Schmidt AM (2003) Glycation, Inflammation and RAGE–a scaffold for the macrovascular complications of diabetes and beyond. Circ Res 93:1159–1169
Acknowledgments
This work was supported by the Interdisciplinary Centre of Clinical Research (IZKF) at the Faculty of Medicine of the University of Leipzig (01KS9504, Project N1), the Alzheimer Forschungs Initiative e.V. (Project 01805), the Deutsche Forschungsgemeinschaft (DFG) (Project MU 1011/14-1) and the Studienstiftung des deutschen Volkes (to KB). We thank Prof. Dr. Thomas Henle und Dr. Bernd Weigele, TU Dresden, Germany for providing the IgG antiRAGE antibody, Dr. Carol Lovelidge for revising the manuscript, and special thanks go to Dr. Jovana Gasic-Milenkovic for all the help that she gave us in all AGE related questions as long as we had the pleasure of working together.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bigl, K., Gaunitz, F., Schmitt, A. et al. Cytotoxicity of advanced glycation endproducts in human micro- and astroglial cell lines depends on the degree of protein glycation. J Neural Transm 115, 1545–1556 (2008). https://doi.org/10.1007/s00702-008-0126-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-008-0126-4