Abstract
Background
External ventricular drain (EVD) placement is a frequently performed neurosurgical procedure. Inaccuracies in drain positioning and the need for multiple passes using the classic freehand insertion technique is well reported in the literature, especially in the traumatic brain injury (TBI) population. The purpose of this study was to evaluate if electromagnetic neuronavigation guidance for EVD insertion improves placement accuracy and minimizes the number of passes in severe TBI patients.
Methods
Navigation was applied prospectively for all new severe TBI patients who required ventricular catheter placement over a period of 1 year, and compared with a retrospective cohort of severe TBI patients who had EVD inserted freehand in the preceding year. The placement accuracy was evaluated using the Kakarla grading system; the number of passes was also compared.
Results
Fifty-four cases were recruited: 35 (64.8%) had their EVD placed using the freehand technique and 19 (35.2%) using navigation guidance. In the navigation group, the placement accuracy was: 94.7% (18/19) grade 1, 5.3% (1/19) grade 2, and none at grade 3. In comparison, freehand placement was associated with misplacement (grades 2 and 3) in 42.9% of the cases (p value = 0.009). The number of passes was significantly lower in the navigation group (mean of 1.16 ± 0.38), compared with the freehand group (mean of 1.63 ± 0.88) (p value = 0.018).
Conclusions
Using the navigation to guide EVD placement was associated with a significantly better accuracy and a lower number of passes in severe TBI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
External ventricular drain (EVD) placement is one of the most commonly performed neurosurgical procedures: it is potentially lifesaving when draining hydrocephalus or when used to manage high intracranial pressure (ICP). This is often performed in the intensive care unit (ICU) and it is one of the first procedures to be learned and performed by neurosurgery trainees [19, 40]. Although EVD insertion is a low morbidity procedure, there are risks involved when passing the drain through vital brain structures in addition to potential injury to vascular injuries [5, 15, 22, 35, 39]. In addition, multiple passes may cause unnecessary brain insult with each additional pass.
Classically, EVD placement is a blind procedure that is performed using freehand technique through a frontal approach utilizing anatomical surface landmarks. Inaccuracy of EVD placement using the freehand technique has been reported in the literature (Table 1) [1, 2, 3, 11, 14, 16,17,18,19, 24, 26, 29,30,31,32, 38, 41, 45]. In their cohort, mostly of subarachnoid hemorrhage (SAH) and intracerebral hemorrhage (ICH), Toma et al. [45] reported the misplacement within the brain parenchyma, subarachnoid space and contralateral side to be 23%. Of the misplaced catheters, 40% required revision or reinsertion. A similar result of high rates of misplacement (23%) was reported by Lee et al. [24] in a Korean cohort that was mostly composed of SAH and ICH patients. Hsieh et al. [17] reported misplacement to be more than 28%, of which traumatic brain injury (TBI) patients constituted about 30% of the sample. The rate of misplacement was significantly higher in patients whose head computed tomography (CT) scans revealed lower hydrocephalus ratio [8] and smaller ventricular size [17, 45].
The number of passes was also reported in some series. In their retrospective study, Phillips et al. [32] reported that multiple passes occurred in 28% of EVD insertions using freehand technique, with an average of 1.85 passes per procedure. In a mixed cohort of EVD insertions including 50% traumatic brain injury (TBI patients), Huyette et al. [18] reported 40% incidence of misplacement of the drain using the freehand method, and more importantly, 20.4% of the catheter tips terminated outside the ventricular system. Furthermore, the number of passes was reported in 30% of the cases and was on average 2.17 (range, 1–5) passes per successful EVD. Severe TBI is currently one of the most common indications for EVD placement for intracranial pressure monitoring and simultaneous CSF drainage [4, 40], but it also represents the most challenging group in terms of accurate EVD placement.
Various techniques have been described to improve ventricular catheter placement. Thirty years ago, Ghajar [14] published his technique using the Ghajar Device (Neurodynamics, New York, NY, USA) to improve the accuracy of EVD placement. The application of navigation-guidance was evaluated for permanent ventricular shunt placement and EVD insertion [10, 26, 40]. In a retrospective cohort study comparing freehand, stereotactic-guided and ultrasound-guided ventricular catheter placement for cerebrospinal fluid (CSF) shunting, the only risk factor identified for placement inaccuracy was the use of freehand technique [46].
Computer-assisted navigation is an image-guided stereotactic technique used to provide localization in surgical procedures. There are four different mechanisms to track medical instruments: mechanical, acoustic, electromagnetic, and optical [43]. Navigation using optical tracking systems is the most widely used image guidance technique in the field of neurosurgery. Limitations include complex set-up, the space required in the operating room, and in most instances, the need for rigid skull fixation. The physical limitations restrict its use for EVD insertion at the bedside. Electromagnetic tracking systems have a simpler setup, and allow freedom of head movement without loss of accuracy of registration. Electromagnetic technology does not result in interference with the surgical field [12, 16, 20, 21, 36]. These advantages make it applicable for urgent EVD placement at the bedside in the ICU.
In our study, we wanted to evaluate the usefulness of neuronavigation to guide EVD placement in severe TBI patients We hypothesized that navigation will improve the accuracy and minimize the number of catheter passes required for successful placement. Our targeted patient population (severe TBI) was based on the notion that this population has less favorable clinical features: young age with small ventricles, brain edema and resultant ventricular compartment effacement. Other unfavorable characteristics that make EVD placement more challenging in this population are the associated brain shift and anatomical distortion, and often the presence of external cranial swelling or lacerations.
Methods
Study design
This was a prospective study to evaluate the accuracy of EVD tip placement using the electromagnetic navigation system (navigation-guidance group). We also compared the results of this approach to a retrospective cohort where the traditional freehand technique for catheter placement was used (freehand group). The study was conducted at the Montreal General Hospital, which is one of the two level-1 trauma centers for adults in the region of Montreal, Quebec, Canada. The retrospective freehand group was collected from patients treated between July 2013 and August 2014. The navigation guidance group was recruited between September 2014 and August 2015.
The primary outcome was the accuracy of catheter tip placement using navigation guidance compared with the freehand technique. Kakarla et al.’s [23] grading was used for this evaluation (Table 2). The other main outcome was to evaluate the multiple passes associated with each procedure. Secondary outcomes included rates of revision, infection and complications, the length of hospital stay, in addition to evaluating the extra time added by the navigation set-up and registration for the navigation group.
This research study was approved by the Research Ethics Board (REB) of the McGill University Health Centre, and approval to conduct the study was also obtained from the hospital administration. For this type of study formal consent is not required.
Patients and data collection
The population of our study included all admitted adult patients (≥18 years old) with severe TBI who required EVD insertion in the ICU for ICP monitoring and treatment, as per the recommendations of the Brain Trauma Foundation [5]. Patients who did not have an EVD insertion, had a parenchymal monitoring device inserted instead of an EVD, and who were subjected to emergency cranial surgery and had an EVD placed intraoperatively were excluded. Patients who had no CT scan post EVD procedure were also excluded. Our institution maintains a database containing demographics (age, gender), cause of injury and severity (Glasgow Coma Scale Score [GCS]), and outcome. This database was the source for identifying the retrospective cases for our study. Other collected data included ICU and hospital length of stay, the need for surgical decompression, the level of training of the person performing the procedure, the documented number of EVD passes, and the functional outcome using the Extended Glasgow Outcome Scale (GOSE) at discharge from the acute care hospital, and whether the discharge destination was home or another medical facility. The GOSE was always assigned according to a consensus within the multidisciplinary team at the time of discharge from the acute care hospital.
CT protocol
Patients enrolled in the study were not subjected to any additional CT scans, avoiding unwanted delays and increased radiation dose. The CT that was used for the first evaluation in the emergency room (ER) and as a part of the patient’s standard of care was used (LightSpeed VCT, 64 slices; axial slices acquisition; slice thickness, 2.5 mm; gantry, zero tilt; GE Healthcare, Little Chalfont, UK).
The neuronavigation system
The StealthStation AxiEM electromagnetic (EM) navigation (Medtronic Navigation, Louisville, CO, USA) is a computer-aided, frameless image-guided stereotactic navigation system used for EVD placement. It employs a portable EM localization system to track instruments and patient anatomy simultaneously. In each procedure, this EM emitter is positioned near the head of the patient and delivers a magnetic field of small intensity, which is used to induce a current on small coils located on instrumentation used in the surgical field. These instrumentations include tools such a tracer pointer to provide location on the patient’s skin, AxiEM stylet to provide location of the catheter tip, and a non-invasive patient tracker to provide the positioning of the head. This system provides real-time position of the instrumentation, relative to two-dimensional (2-D) and three-dimensional (3-D) visualization of the medical imaging obtained prior to the procedure.
In order to evaluate the time required to use this approach, we calculated the total time (navigation time) required to set up the system for navigation tracking; transfer of medical images into the navigation system, and planning the target. We also determined the interval times, registration time and procedure time, required to co-register the uploaded CT scan in the AxiEM system to the patient’s head to be able to simulate real-time catheter placement, and time from the skin incision until skin closure, respectively.
The EVD insertion procedure
Classic freehand technique
The standard practice in our hospital was to place EVDs at the bedside in the ICU. Classically, the scalp incision was made over Kocher’s point, which is about 10 mm anterior to the coronal suture in the mid-pupillary line and about 2.5 cm from midline, and then the periosteum was separated from the skull. The skull was drilled using a manual twist drill The stylet-loaded ventricular catheter was inserted using external landmarks while maintaining an orthogonal trajectory with respect to the skull. The success or failure of freehand EVD placement is measured by the free flow of CSF from the distal end of the catheter.
Navigation technique
The same standard procedure for the preparation, incision and catheter used in the classic freehand approach was taken in the navigation group. The following additional steps for landmarking and trajectory planning were required for the neuronavigation setup. The CT images were uploaded form the hospital picture archiving and communication system (PACS) to the AxiEM electromagnetic neuronavigation system. The setup involved building the 3-D navigation model of the patient’s skin anatomy and selecting the trajectory of the catheter. The planning of the target point was a crucial part of the procedure; we selected a location close to the ipsilateral foramen of Monro as the target. The registration took place when the patient arrived in ICU by using the surface registration over the face and forehead. After confirming the accuracy, the procedure advanced in steps similar to the ones followed in the classic EVD placement. The step of catheter insertion is solely done under the navigation guidance using the navigation stylet-loaded ventricular catheter towards the preselected target close to the foramen of Monro. After successful placement of the EVD, the procedure continued as in the classic technique.
Radiological evaluation
A CT scan was done within 24 h after the EVD insertion, as per standard of care, to verify the catheter tip and to exclude complications. Post procedure CT scans of a mixed cohort of the freehand group and navigation group were used for EVD tip accuracy evaluation. A neuroradiologist blinded to the patients’ details and insertion technique reviewed the scans and classified the accuracy of insertion according to the Kakarla grades of EVD tip accuracy. Radiological complications of catheter insertion were also documented, including any new hemorrhage related to the EVD path. On the pre-procedural scan, other measurements were calculated, including bifrontal ventricular width, hydrocephalus ratio, frontal ventricular size, caudate-septal line, bicaudate width and bicaudate index (Fig. 1). The midline shift was also measured on pre-procedural scans, in addition to evaluating the CT scan structural appearance according to the Marshall grades [30]. Other radiological measures on post-insertion scans were evaluated, including EVD catheter entry point evaluation, the eloquent areas traversed by the catheter (including the corpus callosum). The length of the EVD inserted was measured from the outer table of the cranium at the burr hole to the tip of the catheter, using coronal image reformatted obliquely and centered at the catheter.
Axial computed tomography images showing different measurements used to evaluate scans prior to ventricle catheter insertion. a Hydrocephalus ratio (the ratio of maximum width of the frontal horns to the maximum width of the inner table of the cranium) and bifrontal ventricular width (the maximum width of the frontal horns). b Ventricular size (the width of the ipsilateral frontal horn calculated with a line tangential to the caudate nucleus). c Caudate-septal line (in the ipsilateral side, the length forms a tangential line to the caudate nucleus to the septum pellucidum in the midline). d Bicaudate width (the maximum width between two lines drawn tangential to both caudate nuclei and perpendicular to the septum pellucidum in the midline), and bicudate index (the ratio of the width of both lateral ventricles at the level of the head of caudate nuclei to the maximum width of the inner tables of the cranium at the same axial level)
Clinical evaluation
The number of passes was evaluated as recorded in the procedure note during the chart review. The number of days the catheter remained in situ was entered into the database. The total hospital stay included the number of days from admission to discharge and the ICU stay indicated the number of days initially spent in the ICU, excluding other readmissions. The intubation period included the time from admission to extubation, and in cases of tracheostomy this included the time until the patient was weaned off the ventilator. Patients who were eventually considered of guarded prognosis and subjected to extubation and comfort care measures were excluded from the evaluation of the ICU stay and intubation period. Management of a misplaced EVD was based on clinical grounds; patients with functioning EVD with the tip in an undesirable position were followed as long as the EVD was functional. Malfunctioning EVDs included those documented to not be draining or blocked. CSF infection was categorized into three groups: (1) no infection, (2) contamination as per positive CSF but no clinical signs of CSF infection and (3) infection as per positive CSF, with appropriate clinical picture, requiring intravenous antibiotic therapy. The discharge status included four parameters: (1) home, (2) discharge to a rehabilitation center, (3) transfer to a long-term care facility which included also the transfer to another hospital while awaiting placement at the long-term care facility, and (4) death when it occurred during the same admission.
Statistical analysis
All statistical analyses were performed using SPSS software (IBM SPSS, Statistics version 20.0 for Mac OS; SPSS, Chicago, IL, USA). We reported median or mean (± standard deviation) for continuous variables, and count (%) for categorical variables. Two groups were compared using Student’s independent t-test for continuous variables, and the Chi-square test was used to compare groups for categorical variables. However, Fisher’s exact test was used when more than one expected cell frequency was less than 5 in 2 × 2 contingency table. To compare more than two groups, we used the ANOVA F-test and performed multiple comparisons using the Bonferroni method to maintain the type I error.
Results
There were 132 adult patients admitted with an initial diagnosis of severe TBI during the study’s 2-year period. Figure 2 illustrates the process of selection. Thirty patients were not included because they had no ICP monitor inserted. These were either patients who showed clinical improvement on admission and frequent evaluation was feasible with no signs supporting elevated ICP, or patients who were deemed of guarded outcome prognosis or having evolving brain stem death, and their management was focused on palliative measures. Out of the remaining 102 cases, there were 14 additional revision procedures giving a result of 116 total ICP monitors placed during the study period. The EVD cases that were inserted exclusively intraoperatively (49 cases) were not included; likewise, ten cases of parenchymal ICP monitors were excluded. It is of interest to note that out of the ten cases of parenchymal ICP monitors, three were cases of failed freehand EVD insertion and one was a case with an anticipated EVD insertion difficulty. The eligible cases for evaluation in our study were 57 cases of EVD placement. Three cases were excluded from the retrospective group: because of failed insertion and consequently no post procedural scan available for evaluation (two cases), and in one case the patient died with no follow-up CT scan. Ventricular catheter insertion failure is a valid outcome; however, these cases were excluded as the primary outcome necessitated reviewing the post placement scan. There were two cases that crossed over from the navigation group and were evaluated under the freehand group: one had a CT scan done without using the CT navigation protocol and one had failed the registration because of the late decision of EVD placement (5 h post the CT scan) and that particular patient developed increasing right periorbital and forehead swelling that markedly impaired registration. The final number of procedures included in the study was 54: 35 (64.8%) were placed freehand and 19 (35.2%) with navigation guidance.
Baseline characteristics
Baseline characteristics of the study population are provided in Table 3. A total of 50 patients underwent 54 EVD placement procedures using either the classic freehand technique (n = 34 patients for 35 procedures) or the navigation guidance method (n = 18 patients for 19 procedures), including two patients who had their initial EVD inserted using the freehand technique and then revised because of malfunction using the navigation guidance technique.
The baseline demographics and severity of head injury were comparable between both groups. Table 4 shows the different mode of injuries, and fall was found to be the most common type of injuries for both groups.
Potential determinants of EVD placement accuracy
There are many factors that can affect EVD placement accuracy. Inserting a ventricular catheter using the freehand technique may be the only significant risk factor for misplacement, as found in previous studies. [19, 46] In our study, we included the factors that had been previously reported in the literature and the ones that may have more clinical impact in this determination (Table 5). Large ventricular measurements may indicate a favorable case for catheter placement accuracy in which the target is physically larger. These factors were comparable for the freehand and the navigation groups, except for frontal horn size that was significantly smaller in the navigation group.
EVD accuracy
The results in Table 6 show a significant (p value = 0.009) association between use of navigation guidance and having an optimal and adequate EVD placement (grade 1). Out of 19 cases in the navigation group, 18 (94.7%) were classified as grade 1 accuracy, 1 case (5.3%) as grade 2, and none as grade 3. The grade 2 accuracy case had the ventricular catheter crossed to the contralateral frontal horn (see Fig. 3). The classic freehand group had optimal placement in 57.1% of the cases (grade 1) and suboptimal placement in eloquent tissue was in 37.1% (grade 3). Examples of grade 3 misplacement included the internal capsule, the basal cisterns, the thalamus and brainstem (see Fig. 4). Navigation was also associated with a safe path for EVD insertion (one that does not pass through the corpus callosum basal ganglia, thalamus, pre/post central gyri, brainstem, or internal capsule). Safe path of insertion was seen in 84.2% in the navigation group compared to 42.9% in the freehand group (p value = 0.03).
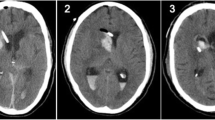
Axial computed tomography images of the single case that had ventricular catheter misplaced into the contralateral frontal horn (grade 2) in the navigation-guided group. A 76-year-old man on antiplatelet therapy who sustained head trauma after a fall. He was operated on urgently for subdural hematoma evacuation and removal of bone flap (a). He had the right ventricular catheter inserted intraoperatively and because of catheter malfunction, he had the left catheter inserted under the navigation guidance. Because of the expansion injury post operatively, he had a large intracerebral hemorrhage with septum pellucidum shifted to the contralateral side (b and c). The catheter tip ended near the contralateral foramen of Monro (d)
Number of passes
The recorded number of passes was unavailable in 23% of the freehand cases but available for 100% of the navigation group. The number of passes was significantly lower in the navigation group, with a mean of 1.16 ± 0.38 (p value = 0.018, 95% CI [−0.86, −0.09]). The freehand group’s mean number of passes was 1.63 ± 0.88, and for the entire cohort it was 1.43 ± 0.75 passes. The two excluded cases from the retrospective cohort (due to failed insertion) were recorded to have two and three passes. Evaluating the number of passes in relation to the placement accuracy revealed that the mean number of passes was statistically lower in grade 1 accuracy (1.25 ± 0.51), compared to grade 3 (1.91 ± 1.04), (F[2, 45] = 5.27, p value = 0.009). Multiple passes were also associated with increasing risk of radiologically documented complications. Indeed, the mean number of passes in the procedures that had been reported to have hemorrhagic complications post insertion was 2.00 ± 1.10 compared to 1.26 ± 0.51 (p value = 0.051, 95% CI [−0.005, 1.491]).
Clinical outcomes
There was a minor tendency towards having better clinical outcomes in the navigation group in comparison to the freehand group. The variables included the time from insertion to removal, ICU admission duration, total hospital admission duration, total days of intubation, risk of EVD malfunction, risk of revisions, risk of infection, radiological complications and death as a final outcome. None of these secondary outcomes were significantly different between the two groups (p > 0.05).
Navigation time
The mean time for the navigation setup was 17.22 ± 6.73 min, and for registration was 17.17 ± 11.05 min. The combination of these two times was considered the additional time required for navigation. However, the navigation setup mean time of 17.22 min was almost always completed prior to the patients’ arrival in their beds in the ICU. The registration mean time of 17.17 min was the actual time added to the procedure to complete the new technique, as it can be done only once the patient is physically in ICU. The mean procedure time from skin opening to closure for the navigation group was 28.67 min.
Discussion
This first study on EVD placement accuracy in TBI patients demonstrates a significant improvement in insertion accuracy when using navigation guidance. Freehand EVD insertion technique using superficial anatomical landmarks currently remains the method of choice due to its simplicity, and more importantly, its efficiency. This remains the most common method practiced by neurosurgeons and is currently the standard of care to access ventricular compartments. Using the ipsilateral medial canthus trajectory was found to be an unreliable guide for directing an EVD, whereas both the perpendicularity to skull and contralateral medial canthus trajectories were significantly more reliable methods for targeting the frontal horn of the ipsilateral lateral ventricle [28]. In another study using a virtual radiological analysis of 3-D data of skull and ventricular anatomy of randomly selected patients with normal ventricular anatomy, Rehman et al. [34] reported 32% misplacement using virtual ventriculostomy trajectories at a perpendicular angle to the skull at Kocher’s point. Additionally, scalp or forehead swelling associated with TBI and intracranial pathology including cerebral shift and edema can contribute to the challenge of the freehand insertion technique.
Ventricular catheter misplacement in eloquent brain tissue can result in significant morbidities that may have very serious consequences and necessitate further interventions [5, 15, 22, 35, 39]. The misplaced catheters may require revision, thereby losing the benefit of therapeutic drainage, involve additional cost and time of repeat CT scan and procedures, and the additional risk of further brain injury. Each insertion pass results in more injuries to the already traumatized brain. An increase in the number of catheter passes is associated with a small but definite risk of complications, including hemorrhage, neurological injury, and infection. Post-procedure imaging studies, such as CT or MRI, will often show effects of iatrogenic trauma from the catheter’s insertion. There may or may not be associated clinically detectable findings from multiple catheter placement attempts. Subtle neuropsychological effects may be present even when no clinically detectable effect is found. Hence, the number of passes should be minimized.
Methods reported to improve EVD placement
Different methods have evolved to improve the accuracy of EVD placement. Ghajar Guide application, when introduced, was studied in 17 patients with a good success rate on the first pass. Only 11 patients had confirmation of accurate placement using fluoroscopy [14]. The efficacy of the Ghajar Guide was confirmed in a prospective study [30] Using the same simple principle of placing a device to the skull to guide a perpendicular catheter insertion, Yamada et al. [47] introduced a tripod to help ventriculo-peritoneal shunt insertion and found significantly better catheter tip accuracy when comparing the tripod method with freehand insertion. Again, the placement was not ideal in about 54% of the freehand group. Similarly, a study applied the principle of having the ventricular drain insertion trajectory perpendicular to the skull, and used a smartphone application to determine the angulation of the catheter can help ventricular catheter placement. Most of these cases were done for the management of hydrocephalus in neonates [44].
It should be noted that using the Ghajar Guide principle is useful only when the patient’s anatomy has not been distorted and favors patients with large-size ventricles. In trauma, it is common to have scalp lacerations or contusions that may distort the perpendicular plane over the burr hole in addition to the intracranial anatomical distortions and brain shift.
Image-guided methods of ventriculostomy catheter placement might minimize the number of passes and improve accuracy. Ultrasound guidance, CT scan and live fluoroscopic CT navigation guidance were used in small numbers of case series with good placement accuracy and success from a single pass [6, 9, 23, 33, 37]. Endoscopic, stereotaxic and robotic ventricular catheter placement methods have been described to improve accuracy in ventriculo-peritoneal shunt insertion procedures [7, 10, 13, 25, 46]. The feasibility of these methods is limited in urgent cases of severe TBI, and they require specific settings that may not be available outside the operating room (OR).
The field of navigation continues to evolve and new tools are being developed to make catheter placement easier, safer and more accurate [42]. In a prospective study by Mahan et al. [26] using the frameless electromagnetic navigation for EVD placement, accuracy was 94.1%.
Novelty of our study and limitations
The novelty of our study is the targeted population of severe TBI who were repeatedly reported in the literature to have high EVD misplacement rates. This is the first study to prospectively test the use of this innovative technology to guide EVD placement in severe TBI. The population of both groups in our study was from the same institution, sharing the same conditions and indications for catheter placement. The post procedure CT scan evaluation was performed by a blinded neuroradiologist, who was shown a set of cases randomized from both patient groups (navigation and freehand).
The cost of the equipment used in this study is more expensive in the navigation approach when compared with freehand placement, and requires equipment that may be complex to use, and require dedicated space for storage and use. Also, the navigation systems have limited capability for real-time guidance, rendering them of questionable accuracy in case of ongoing anatomical distortion between the time of the scan and the time of procedure.
There was an added time to set up the navigation system, but it was relatively short. The lack of a reliable procedure time record in the retrospective group made it difficult to reach a conclusion in regards to the extra time used for the setup and registration and the overall time difference for the whole procedure. The freehand method, which often requires multiple passes, may actually lengthen the procedure time, and misplacement may involve an additional procedure for revision, additional scans and cost.
The advantage of accurate placement and smaller number of passes was evident in our study which may lead to improved efficiency as well as improved safety. The modest improvement in revision/malfunction rates and radiological complications, can lead to benefits in a large cohort of patients.
The ideal ventricular catheter placement would be one that: (1) has an optimal entry site into the skull, (2) has a safe path and distal tip ending at the targeted location close to the foramen of Monro, (3) requires one single pass, $4) is deemed functional with no post insertion complications, (5) has short procedure time, and (6) necessitates no additional cost.
Conclusion
Chasing perfection in EVD placement involves placement accuracy, and this is not currently attainable using the standard blind freehand technique, with its inherent common risks of misplacement and multiple passes. Despite adequate training and experience, ventricular catheter misplacement does occur frequently in severe TBI. Although many neurosurgeons believe that the current practice of freehand placement of ventricular catheter is good enough, the results of this study show that there is certainly much room for improvement. The easy-to-use, accurate bedside-use electromagnetic navigation guidance system is an innovative technique to guide ventricular catheter placement in severe TBI, and has the potential to reduce morbidities associated with this procedure. The electromagnetic navigation system to improve EVD placement accuracy is available, feasible, and accurate. Indication for its use can be strongly supported when EVD placement is predicted to be difficult as in TBI with brain swelling, compressed, small ventricles and midline shift.
References
Abdoh MG, Bekaert O, Hodel J, Diarra SM, Le Guerinel C, Nseir R et al (2012) Accuracy of external ventricular drainage catheter placement. Acta Neurochir (Wein) 154:153–159
Anderson RC, Kan P, Klimo P, Brockmeyer DL, Walker ML, Kestle JR (2004) Complications of intracranial pressure monitoring in children with head trauma. J Neurosurg 101:53–58
Bogdahn UU (1992) Continuous-pressure controlled, external ventricular drainage for treatment of acute hydrocephalus—evaluation of risk factors. Neurosurgery 31:898–903
Brain Trauma Foundation, American Association of Neurological Surgeons (AANS), Congress of Neurological Surgeons (CNS), AANS/CNS Joint Section on Neurotrauma and Critical Care, Bratton SL et al (2007) Guidelines for the Management of Severe Traumatic Brain Injury, VI. Indications for intracranial pressure monitoring. J Neurotrauma 24(Suppl 1):S37–S44
Chai FY, Farizal F, Jegan T (2013) Coma due to malplaced external ventricular drain. Turk Neurosurg 23:561–563
Cooke DL, Levitt M, Kim LJ, Hallam DK, Ghodke B (2011) Transcranial access using fluoroscopic flat panel detector CT navigation. AJNR Am J Neuroradiol 32:E69–E70
Crowley RW, Dumont AS, Asthagiri AR, Torner JC, Medel R, Jane JA Jr et al (2014) Intraoperative ultrasound guidance for the placement of permanent ventricular cerebrospinal fluid shunt catheters: a single-center historical cohort study. World Neurosurg 81:397–403
Evans WA Jr (1942) An encephalographic ratio for estimating the size of the cerebral ventricles: further experience with serial observations. Am J Dis Child 64:820–830
Fiorella D, Peeling L, Denice CM, Sarmiento M, Woo HH (2014) Integrated flat detector CT and live fluoroscopic-guided external ventricular drain placement within the neuroangiography suite. J Neurointerv Surg 6:457–460
Flannery AM, Duhaime AC, Tamber MS, Kemp J, Pediatric Hydrocephalus Systematic R, Evidence-Based Guidelines Task F (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 3: endoscopic computer-assisted electromagnetic navigation and ultrasonography as technical adjuvants for shunt placement. J Neurosurg Pediatr 14(Suppl 1):24–29
Foreman PM, Hendrix P, Griessenauer CJ, Schmalz PGR, Harrigan MR (2015) External ventricular drain placement in the intensive care unit versus operating room: evaluation of complications and accuracy. Clin Neurol Neurosurg 128:94–100
Franz Alfred MA (2014) Electromagnetic tracking in medicine—a review of technology, validation, and applications. IEEE Trans Med Imaging 33:1702–1725
Gautschi OP, Smoll NR, Kotowski M, Schatlo B, Tosic M, Stimec B et al (2014) Non-assisted versus neuro-navigated and XperCT-guided external ventricular catheter placement: a comparative cadaver study. Acta Neurochir (Wein) 156:777–785 discussion 785
Ghajar J (1985) A guide for ventricular catheter placement. J Neurosurg 63:985–986
Grandhi R, Zwagerman NT, Lee P, Jovin T, Okonkwo DO (2015) Iatrogenic pseudoaneurysm of the middle meningeal artery after external ventricular drain placement. J Neuroimaging 25:140–141
Hayhurst C, Byrne P, Eldridge PR, Mallucci CL (2009) Application of electromagnetic technology to neuronavigation: a revolution in image-guided neurosurgery. J Neurosurg 111:1179–1184
Hsieh CT, Chen GJ, Ma HI, Chang CF, Cheng CM, Su YH et al (2011) The misplacement of external ventricular drain by freehand method in emergent neurosurgery. Acta Neurol Belg 111:22–28
Huyette DR, Turnbow BJ, Kaufman C, Vaslow DF, Whiting BB, Oh MY (2008) Accuracy of the freehand pass technique for ventriculostomy catheter placement: retrospective assessment using computed tomography scans. J Neurosurg 108:88–91
Kakarla UK, Chang SW, Theodore N, Spetzler RF, Kim LJ (2008) Safety and accuracy of bedside external ventricular drain placement. Neurosurgery 63:ONS162–ONS167
Kandasamy J, Hayhurst C, Clark S, Jenkinson MD, Byrne P, Karabatsou K et al (2011) Electromagnetic stereotactic ventriculoperitoneal csf shunting for idiopathic intracranial hypertension: a successful step forward? World Neurosurg 75:155–160 discussion 132–153
Koivukangas T, Katisko JP, Koivukangas JP (2013) Technical accuracy of optical and the electromagnetic tracking systems. Springerplus 2:90
Kosty J, Pukenas B, Smith M, Storm PB, Zager E, Stiefel M et al (2013) Iatrogenic vascular complications associated with external ventricular drain placement: a report of 8 cases and review of the literature. Neurosurgery 72:208–213 discussion 213
Krotz M, Linsenmaier U, Kanz KG, Pfeifer KJ, Mutschler W, Reiser M (2004) Evaluation of minimally invasive percutaneous CT-controlled ventriculostomy in patients with severe head trauma. Eur Radiol 14:227–233
Lee JH, Park CW, Lee U, Kim YB, Yoo CJ, Kim EY et al (2010) Accuracy of the free hand placement of an external ventricular drain (EVD). Korean J Cerebrovasc Surg 12:82–86
Lollis SS, Roberts DW (2009) Robotic placement of a CNS ventricular reservoir for administration of chemotherapy. Br J Neurosurg 23:516–520
Mahan M, Spetzler RF, Nakaji P (2013) Electromagnetic stereotactic navigation for external ventricular drain placement in the intensive care unit. J Clin Neurosci 20:1718–1722
Marshall LF, Marshall SB, Klauber MR, Clark MB, Eisenberg HM, Jane JA et al (1991) A new classification of head injury based on computerized tomography.J Neurosurg 75:S14–S20
Muirhead WR, Basu S (2012) Trajectories for frontal external ventricular drain placement: virtual cannulation of adults with acute hydrocephalus. Br J Neurosurg 26:710–716
Ngo QN, Ranger A, Singh RN, Kornecki A, Seabrook JA, Fraser DD (2009) External ventricular drains in pediatric patients. Pediatr Crit Care Med 10:346–351
O’Leary ST, Kole MK, Hoover DA, Hysell SE, Thomas A, Shaffrey CI (2000) Efficacy of the Ghajar guide revisited: a prospective study. J Neurosurg 92:801–803
Park YG, Woo HJ, Kim E, Park J (2011) Accuracy and safety of bedside external ventricular drain placement at two different cranial sites: Kocher’s point versus forehead. J Korean Neurosurg Soc 50:317–321
Phillips SB, Delly F, Nelson C, Krishnamurthy S (2014) Bedside external ventricular drain placement: can multiple passes be predicted on the computed tomography scan before the procedure? World Neurosurg 82:739–744
Phillips SB, Gates M, Krishnamurthy S (2012) Strategic placement of bedside ventriculostomies using ultrasound image guidance: report of three cases. Neurocrit Care 17:255–259
Rehman T, Rehman A, Ali R, Rehman A, Bashir H, Ahmed Bhimani S et al (2013) A radiographic analysis of ventricular trajectories. World Neurosurg 80:173–178
Rosenbaum BP, Wheeler AM, Krishnaney AA (2013) External ventricular drain placement causing upgaze palsy: case report. Clin Neurol Neurosurg 115:1514–1516
Rosenow JM, Sootsman WK (2006) Application accuracy of an electromagnetic field-based image-guided navigation system. Stereotact Funct Neurosurg 85:75–81
Ruchholtz S, Waydhas C, Muller A, Lewan UM, Nast-Kolb D, Euler E et al (1998) Percutaneous computed tomographic-controlled ventriculostomy in severe traumatic brain injury. J Trauma 45:505–511
Saladino A, White JB, Wijdicks EF, Lanzino G (2009) Malplacement of ventricular catheters by neurosurgeons: a single institution experience. Neurocrit Care 10:248–252
Schuette AJ, Blackburn SL, Barrow DL, Cawley CM (2012) Pial arteriovenous fistula resulting from ventriculostomy. World Neurosurg 77:785.e781–785.e782
Srinivasan VM, O’Neill BR, Jho D, Whiting DM, Oh MY (2014) The history of external ventricular drainage. J Neurosurg 120:228–236
Stangl AP (1998) Continuous external CSF drainage—a perpetual problem in neurosurgery. Surg Neurol 50:77–82
Stieglitz LH, Giordano M, Samii M, Luedemann WO (2010) A new tool for frameless stereotactic placement of ventricular catheters. Neurosurgery 67:131–135 discussion 135
Stroszczynski C (2006) Minimally invasive tumor therapies. Springer, Berlin Heidelberg
Thomale UW, Knitter T, Schaumann A, Ahmadi SA, Ziegler P, Schulz M et al (2013) Smartphone-assisted guide for the placement of ventricular catheters. Childs Nerv Syst 29:131–139
Toma AK, Camp S, Watkins LD, Grieve J, Kitchen ND (2009) External ventricular drain insertion accuracy: is there a need for change in practice? Neurosurgery 65:1197–1201
Wilson TJ, Stetler WR Jr, Al-Holou WN, Sullivan SE (2013) Comparison of the accuracy of ventricular catheter placement using freehand placement, ultrasonic guidance, and stereotactic neuronavigation. J Neurosurg 119:66–70
Yamada SM, Yamada S, Goto Y, Nakaguchi H, Murakami M, Hoya K et al (2012) A simple and consistent technique for ventricular catheter insertion using a tripod. Clin Neurol Neurosur 114:622–626
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial and material support
Medtronic of Canada Ltd. provided in-kind funding in the form of the StealthStation AxiEM system plus its disposable kits, each containing a registration probe, patient reference device and a sterile navigation stylet. None of the authors received any financial support or any other form of support to conduct this study. The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
This research study was approved by the Research Ethics Board (REB) of the McGill University Health Centre, and approval to conduct the study was also obtained from the hospital administration. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required by our institution.
Funding
Medtronic of Canada Ltd. provided in-kind funding in the form of the StealthStation AxiEM system plus its disposable kits, each containing a registration probe, patient reference device and a sterile navigation stylet. None of the authors received any financial support or any other form of support to conduct this study. The sponsor had no role in the design or conduct of this research.
Rights and permissions
About this article
Cite this article
AlAzri, A., Mok, K., Chankowsky, J. et al. Placement accuracy of external ventricular drain when comparing freehand insertion to neuronavigation guidance in severe traumatic brain injury. Acta Neurochir 159, 1399–1411 (2017). https://doi.org/10.1007/s00701-017-3201-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3201-5