Abstract
Background
Giant intracranial aneurysms (GIA) are often not eligible for direct clip occlusion. Surgical alternatives include partial clip occlusion or the placement of a cerebrovascular bypass or the combination of both. These alternative indirect strategies are expected to lead to a decrease in GIA volume over time rather than instantaneously. To examine whether this is the case, we analyzed follow-up imaging results 1 year after surgery.
Methods
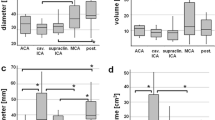
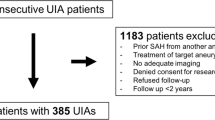
We retrospectively screened the prospective GIA Registry’s imaging database for anterior circulation GIA treated by surgical strategies other than direct clipping. We measured pre- and 1-year post-treatment GIA volume, lateral ventricle volume (LVV), and mid-line shift (MLS) in 19 cases.
Results
After a mean follow-up of 466 days (standard deviation ±171) GIA volumes decreased from 9.6 cm3 (interquartile range (IQR) 6.1–14.1) to 4.3 cm3 (IQR 2.9–5.7; p < 0.01). Ipsilateral LVV increased from 8.6 cm3 (IQR 6.4–24.9) to 16.0 cm3 (IQR 9.1–27.2; p < 0.01) while contralateral LVV increased from 10.3 cm3 (IQR 7.3–20.1) to 11.7 cm3 (IQR 8.2–19.4; p = 0.02). MLS changed from 0.1 mm (IQR −1.9 to 2.0) to −0.9 mm (IQR −1.8 to 0.4; p = 0.03). The decrease in GIA volume correlated with the increase in ipsilateral LVV (rs = 0.60; p = 0.01) but not with the changes in MLS (rs = 0.41; p = 0.08).
Conclusions
In our patient cohort, surgical strategies other that direct clipping for the treatment of anterior circulation GIA lead to a significant decrease in GIA volume over time. The resulting decrease in mass effect was more sensitively monitored by the measurement of changes in ipsilateral LVV than changes in MLS.
Clinical Trial Registration-URL
http://www.clinicaltrials.gov. Unique identifier: NCT02066493.


Similar content being viewed by others
References
Alvarez H (2009) Etiology of giant aneurysms and their treatment. Am J Neuroradiol 30, E8
Anderson RC, Grant JJ, de la Paz R, Frucht S, Goodman RR (2002) Volumetric measurements in the detection of reduced ventricular volume in patients with normal-pressure hydrocephalus whose clinical condition improved after ventriculoperitoneal shunt placement. J Neurosurg 97:73–79
Baschnagel AM, Meyer KD, Chen PY, Krauss DJ, Olson RE, Pieper DR, Maitz AH, Ye H, Grills IS (2013) Tumor volume as a predictor of survival and local control in patients with brain metastases treated with γ knife surgery. J Neurosurg 119:1139–1144
Dengler J, Heuschmann PU, Endres M, Meyer B, Rohde V, Rufenacht DA, Vajkoczy P, Giant Intracranial Aneurysm Study Group (2011) The rationale and design of the Giant Intracranial Aneurysm Registry: a retrospective and prospective study. Int J Stroke 6:266–270
Forsyth PA, Posner JB (1993) Headaches in patients with brain tumors: a study of 111 patients. Neurology 43:1678–1683
Krings T, Alvarez H, Reinacher P, Ozanne A, Baccin CE, Gandolfo C, Zhao WY, Reinges MH, Lasjaunias P (2007) Growth and rupture mechanism of partially thrombosed aneurysms. Interv Neuroradiol 13:117–126
Kulcsár Z, Houdart E, Bonafé A, Parker G, Millar J, Goddard AJ, Renowden S, Gál G, Turowski B, Mitchell K, Gray F, Rodriguez M, van den Berg R, Gruber A, Desal H, Wanke I, Rüfenacht DA (2011) Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol 32:20–25
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198
Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, Berez AL, Tran Q, Nelson PK, Fiorella D (2009) Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery 64:632–642
Nag C, Das K, Ghosh M, Khandakar MR (2012) Prediction of clinical outcome in acute hemorrhagic stroke from a single CT scan on admission. N Am J Med Sci 4:463–467
Pullicino PM, Alexandrov AV, Shelton JA, Alexandrova NA, Smurawska LT, Norris JW (1997) Mass effect and death from severe acute stroke. Neurology 49:1090–1095
Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, FitzMaurice E, Wendell L, Goldstein JN, Greenberg SM, Rosand J (2008) Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 39:2304–2309
Schebesch KM, Proescholdt M, Ullrich OW, Camboni D, Moritz S, Wiesenack C, Brawanski A (2010) Circulatory arrest and deep hypothermia for the treatment of complex intracranial aneurysms–results from a single European center. Acta Neurochir 152:783–792
Schubiger O, Valavanis A, Wichmann W (1987) Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology 29:266–271
Skeie BS, Skeie GO, Enger PØ, Ganz JC, Heggdal JI, Ystevik B, Hatteland S, Parr E, Pedersen PH (2011) Gamma knife surgery in brain melanomas: absence of extracranial metastases and tumor volume strongest indicators of prolonged survival. World Neurosurg 75:684–691
Strik HM, Borchert H, Fels C, Knauth M, Rienhoff O, Bähr M, Verhey JF (2005) Three-dimensional reconstruction and volumetry of intracranial haemorrhage and its mass effect. Neuroradiology 47:417–424
Sughrue ME, Saloner D, Rayz VL, Lawton MT (2011) Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery 69:1261–1270
Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W, Berez A, Nelson PK (2010) Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. Am J Neuroradiol 31:1139–1147
Toma AK, Holl E, Kitchen ND, Watkins LD (2011) Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 68:939–944
Vajkoczy P (2009) Revival of extra-intracranial bypass surgery. Curr Opin Neurol 22:90–95
Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA, Martin NA (2003) Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 60:1441–1446
Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ (1999) Progression of mass effect after intracerebral hemorrhage. Stroke 30:1167–1173
Acknowledgments
The Giant Intracranial Aneurysm Registry is funded by the Center for Stroke Research – Berlin.
Statement
All trials based on data from the Giant Intracranial Aneurysm Registry were approved by the ethics committees of all participating centers and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients included gave their informed consent prior to their inclusion in the study.
Conflict of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Comment
The giant intracranial aneurysm is a complex neurosurgical pathology with extraordinarily high associated morbidity and multiple factors that must be kept in mind during treatment, including both risk of rupture, as well as mass effect, as the authors thoroughly investigate. Through volumetric and linear measurements, the authors utilize the Giant Intracranial Aneurysm Registry to assess the amount of brain compression caused by these aneurysms after treatment by indirect surgical management with or without bypass. Through this retrospective study, with inclusion criteria of at least a 9-month follow-up MRI (although cited in the abstract to be 1 year), the authors correlate decreasing anterior circulation giant aneurysm volume with increasing ipsilateral ventricular volume, thus serving as a measure for the amount of mass effect an aneurysm is imparting onto the surrounding brain and potentially a proxy for aneurysmal involution, which may be obscured on imaging due to metallic artifact post-treatment.
While the authors pose an interesting question, the clinical application of this data is not thoroughly explored, although it is noted that the outcomes will be discussed in another publication. Given that the average modified Rankin scale prior to treatment of the studied patient population is less than 1, the mass effect of the aneurysms is clinically negligible pre-procedurally, so monitoring only the radiographic finding has minimal clinical application. In addition to minimal clinical information being reported, no angiographic imaging is reported, and given that decreasing mass effect of giant aneurysms is not necessarily associated with cessation of blood flow into the lesion, it is unclear how the results of this paper lends to conclusions applicable to patient care (Darsaut 2011).
The authors choose to correlate aneurysm volume to midline shift and both ipsilateral and contralateral lateral ventricular volume, citing that these measurements have been correlated to intracranial hemorrhage outcomes in the past. However, it should be kept in mind the difference in the slow-growing pathologies of a giant intracranial aneurysm, especially in the setting of wall calcifications, and that of an acute intracranial hemorrhage. There is an inverse correlation between ipsilateral ventricular volume and aneurysm volume, but additionally, a paradoxical midline shift towards the treated aneurysm should be more thoroughly investigated, because while a 1-mm pre-treatment midline shift is most often clinically insignificant, typically a 9-mm midline shift, which was found in post-treatment analysis, would need to be intervened on in most neurosurgical settings. Additionally, the patients with bilateral aneurysms should be excluded from analysis because of the significant confounding nature of bilateral compressive pathologies on midline shift and contralateral ventricular volume.
Given the small sample size of patients and the heterogeneity of both aneurysm location and treatment approach in this study, computational flow dynamic studies would be valuable in understanding how these complex aneurysms responded to indirect treatment, and could direct future surgical management of these lesions (Lawton 2011). However, to that end, the likely future of treatment of these highly morbid lesions, often necessitating surgical intervention shown to be fraught with potential for clinical and neurologic complications, will likely be exclusively endovascular, since the advent of the flow-diverting stent. Although the specific locations of these aneurysms are not discussed in this study, it is likely that many, if not all, of the internal carotid artery lesions could have been treated via flow-diverting stent constructs, with not only exceptional aneurysmal occlusion outcomes but also with even more decrease in aneurysmal volume than seen in this trial (Szikora 2013).
As endovascular therapies, including flow-diverter stents, continue to evolve, they will likely become the preferred treatment for not only giant internal carotid artery aneurysms but also for more distal anterior circulation, as well as posterior circulation, aneurysms, where the obstacles related to flow demand to perforators will likely be overcome with further innovation. As with indirect bypass, flow diverters aim for endoluminal remodeling, rather than abrupt aneurysmal occlusion, so involved blood vessels may either maintain patency or develop collateral perfusion, as seen in the ophthalmic artery aneurysm flow-diversion experience (Zanaty 2015).
The authors are to be commended on their thorough objective study of giant intracranial aneurysm mass effect. With inclusion of clinical data and angiographic outcomes, and understanding of the implications resultant from paradoxical midline shift towards the treated aneurysm, this paper will be a fine addition to the literature. As giant aneurysms treated with flow diverters are added to the Giant Intracranial Aneurysm Registry, this paper will serve as a resource on how to compare radiographic outcomes of this pathology with a changing treatment paradigm in the future.
Daniel M. Heiferman, Dustin M. Hayward, Christopher M. Loftus
Illinois, USA
References
Darsaut TE, Darsaut NM, Chang SD, Silverberg GD, Shuer LM, Tian L, Dodd RL, Do HM, Marks MP, Steinberg GK (2011) Predictors of clinical and angiographic outcome after surgical or endovascular therapy of very large and giant intracranial aneurysms. Neurosurgery 68:903–915
Sughrue ME, Saloner D, Rayz VL, Lawton MT (2011) Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery 69:1261–1270
Szikora I, Marosfoi M, Salomváry B, Berentei Z, Gubucz I (2013) Resolution of mass effect and compression symptoms following endoluminal flow diversion for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol 34:935–939.
Zanaty M, Chalouhi N, Barros G, Schwartz EW, Saigh MP, Starke RM, Whiting A, Tjoumakaris SI, Hasan D, Rosenwasser RH, Jabbour P (2015) Flow-diversion for ophthalmic segment aneurysms. Neurosurgery 76:286–290.
No parts of this project have been presented anywhere before.
Rights and permissions
About this article
Cite this article
Maldaner, N., Guhl, S., Mielke, D. et al. Changes in volume of giant intracranial aneurysms treated by surgical strategies other than direct clipping. Acta Neurochir 157, 1117–1123 (2015). https://doi.org/10.1007/s00701-015-2448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2448-y