Abstract
Background
The agent, 2-mercaptoethane sulfonate (MESNA), is a synthetic small molecule, widely used as a systemic protective agent against chemotherapy toxicity, but is primarily used to reduce hemorrhagic cystitis induced by cyclophosphamide. Because MESNA has potential antioxidant and cytoprotective effects, so we hypothesized that MESNA may protect the brain against traumatic injury.
Method
Thirty-two rats were randomized into four groups of eight animals each; Group 1 (sham), Group 2 (trauma), Group 3 (150 mg/kg MESNA), Group 4 (30 mg/kg methylprednisolone). Only skin incision was performed in the sham group. In all the other groups, the traumatic brain injury model was created by an object weighing 450 g falling freely from a height of 70 cm through a copper tube on to the metal disc over the skull. The drugs were administered immediately after the injury. The animals were killed 24 h later. Brain tissues were extracted for analysis, where levels of tissue malondialdehyde, caspase-3, glutathione peroxidase, superoxide dismutase, nitric oxide, nitric oxide synthetase and xanthine oxidase were analyzed. Also, histopathological evaluation of the tissues was performed.
Results
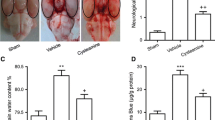
After head trauma, tissue malondialdehyde levels increased; these levels were significantly decreased by MESNA administration. Caspase-3 levels were increased after trauma, but no effect of MESNA was determined in caspase-3 activity. Following trauma, both glutathione peroxidase and superoxide dismutase levels were decreased; MESNA increased the activity of both these antioxidant enzymes. Also, after trauma, nitric oxide, nitric oxide synthetase and xanthine oxidase levels were increased; administration of MESNA significantly decreased the levels of nitric oxide, nitric oxide synthetase and xanthine oxidase, promising an antioxidant activity. Histopathological analysis showed that MESNA protected the brain tissues well from injury.
Conclusions
Although further studies considering different dose regimens and time intervals are required, MESNA was shown to be at least as effective as methylprednisolone in the traumatic brain injury model.



Similar content being viewed by others
References
Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615
Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, Cai J, Pierce WM, Vore M, Moscow JA, St Clair DK, Butterfield DA (2011) 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-α release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med 50:1630–1638
Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S (2007) Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem 294:137–144
Awasthi D, Church DF, Torbati D, Carey ME, Pryor WA (1997) Oxidative stress following traumatic brain injury in rats. Surg Neurol 47:575–581
Berrigan MJ, Marinello AJ, Pavelic Z, Williams CJ, Struck RF, Gurtoo HL (1982) Protective role of thiols in cyclophosphamide-induced urotoxicity and depression of hepatic drug metabolism. Cancer Res 42:3688–3695
Bramlett HM, Dietrich WD (2004) Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab 24:133–150
Diaz-Ruiz A, Rios C, Duarte I, Correa D, Guizar-Sahagun G, Grijalva I, Madrazo I, Ibarra A (2000) Lipid peroxidation inhibition in spinal cord injury: cyclosporin-A vs methylprednisolone. NeuroReport 11:1765–1767
Dolgun H, Sekerci Z, Turkoglu E, Kertmen H, Yilmaz ER, Anlar M, Erguder IB, Tuna H (2010) Neuroprotective effect of mesna (2-mercaptoethane sulfonate) against spinal cord ischemia/reperfusion injury in rabbits. J Clin Neurosci 17:486–489
Durak İ, Kavutcu M, Kaçmaz M, Avcı A, Horasanlı E, Dikmen B, Çimen MYB, Öztürk HS (2001) Effects of isoflurane on nitric oxide metabolism and oxidant status of rat myocardium. Acta Anaesthesiol Scand 45:119–122
Erkan Ustün M, Md AD, Oztin Oğün C, Sümer F, Gürbilek M (2001) Effects of deferoxamine on tissue superoxide dismutase and glutathione peroxidase levels in experimental head trauma. J Trauma 51:22–25
Evans PH (1993) Free radicals in brain metabolism and pathology. Br Med Bull 49:577–587
Gressier B, Lebegue N, Brunet C, Luyckx M, Dine T, Cazin M, Cazin JC (1995) Scavenging of reactive oxygen species by letosteine, a molecule with two blocked-SH groups. Comparison with free-SH drugs. Pharm World Sci 17:76–80
Gressier B, Lebegue S, Brunet C, Luyckx M, Dine T, Cazin M, Cazin JC (1994) Pro-oxidant properties of methotrexate: evaluation and prevention by an anti-oxidant drug. Pharmazie 49:679–681
Hall ED, Braughler JM (1989) Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med 6:303–313
Hille R, Nishino T (1995) Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J 9:995–1003
Ikeda Y, Long DM (1990) The molecular basis of brain injury and brain edema: the role of oxygen free radicals. Neurosurgery 27:1–11
Keane RW, Kraydieh S, Lotocki G, Bethea JR, Krajewski S, Reed JC, Dietrich WD (2001) Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J Neuropathol Exp Neurol 60:422–429
Kobeissy FH, Ottens AK, Zhang Z, Liu MC, Denslow ND, Dave JR, Tortella FC, Hayes RL, Wang KK (2006) Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol Cell Proteomics 5:1887–1898
Kontos HA, Povlishock JT (1986) Oxygen radicals in brain injury. Cent Nerv Syst Trauma 3:257–263
Lotocki G, Alonso OF, Frydel B, Dietrich WD, Keane RW (2003) Monoubiquitination and cellular distribution of XIAP in neurons after traumatic brain injury. J Cereb Blood Flow Metab 23:1129–1136
Lukacova N, Halat G, Chavko M, Marsala J (1996) Ischemia– reperfusion injury in the spinal cord of rabbits strongly enhances lipid peroxidation and modifies phospholipid profiles. Neurochem Res 21:869–873
Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K (1994) A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg 80:291–300
Nishikawa A, Morse MA, Chung FL (2003) Inhibitory effects of 2-mercaptoethane sulfonate and 6-phenylhexyl isothiocyanate on urinary bladder tumorigenesis in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Cancer Lett 193:11–16
Nishio S, Yunoki M, Noguchi Y, Kawauchi M, Asari S, Ohmoto T (1997) Detection of lipid peroxidation and hydroxyl radicals in brain contusion of rats. Acta Neurochir Suppl 70:84–86
Ozdemir D, Uysal N, Gonenc S, Acikgoz O, Sonmez A, Topcu A, Ozdemir N, Duman M, Semin I, Ozkan H (2005) Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol Res 54:631–637
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pineda JA, Wang KK, Hayes RL (2004) Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol 14:202–209
Prajda N, Weber G (1975) Malignant transformation-linked imbalance: decreased XO activity in hepatomas. FEBS Lett 59:245–249
Qian H, Liu D (1997) The time course of malondialdehyde production following impact injury to rat spinal cord as measured by microdialysis and high pressure liquid chromatography. Neurochem Res 22:1231–1236
Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36:1–9
Schwerdt G, Kirchhoff A, Freudinger R, Wollny B, Benesic A, Gekle M (2007) Mesna or cysteine prevents chloroacetaldehyde-induced cell death of human proximal tubule cells. Pediatr Nephrol 22:798–803
Sener G, Sehirli O, Ercan F, Sirvanci S, Gedik N, Kacmaz A (2005) Protective effect of MESNA (2-mercaptoethane sulfonate) against hepatic ischemia/reperfusion injury in rats. Surg Today 35:575–580
Shohami E, Beit-Yannai E, Horowitz M, Kohen R (1997) Oxidative stress in closed-head injury: brain antioxidant capacity as an indicator of functional outcome. J Cereb Blood Flow Metab 17:1007–1019
Southorn PA, Powis G (1988) Free radicals in medicine. II. Involvement in human disease. Mayo Clin Proc 63:390–408
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Tyurin VA, Tyurina YY, Borisenko GG, Sokolova TV, Ritov VB, Quinn PJ, Rose M, Kochanek P, Graham SH, Kagan VE (2000) Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J Neurochem 75:2178–2189
Ucar T, Tanriover G, Gurer I, Onal MZ, Kazan S (2006) Modified experimental mild traumatic brain injury model. J Trauma 60:558–565
Umemura T, Hasegawa R, Sai-Kato K, Nishikawa A, Furukawa F, Toyokuni S, Uchida K, Inoue T, Kurokawa Y (1996) Prevention by 2-mercaptoethane sulfonate and N-acetylcysteine of renal oxidative damage in rats treated with ferric nitrilotriacetate. Jpn J Cancer Res 87:882–886
Ustün ME, Duman A, Oğun CO, Vatansev H, Ak A (2001) Effects of nimodipine and magnesium sulfate on endogenous antioxidant levels in brain tissue after experimental head trauma. J Neurosurg Anesthesiol 13:227–232
Wennersten A, Holmin S, Mathiesen T (2003) Characterization of Bax and Bcl-2 in apoptosis after experimental traumatic brain injury in the rat. Acta Neuropathol 105:281–288
Yakovlev AG, Faden AI (2004) Mechanisms of neural cell death: implications for development of neuroprotective treatment strategies. NeuroRx 1:5–16
Ypsilantis P, Tentes I, Assimakopoulos SF, Kortsaris A, Scopa CD, Simopoulos C (2004) Mesna ameliorates intestinal mucosa damage after ifosfamide administration in the rabbit at a dose-related manner. J Surg Res 121:84–91
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
In this experimental study, the authors try to perform biochemical and histopathological analysis about the neuroprotective effects of MESNA in TBI. The study is well conceived and their results sound to be reasonable. The authors concluded that MESNA may play a role in TBI by reducing lipid peroxidation and increasing antioxidant activity. On the other hand, no antiapoptotic activity of MESNA could be shown. The idea to use a well-known systemic protective agent against chemotherapy toxicity in TBI should be stuck at in order to obtain a clinical therapeutic application in TBI practice.
Alex Alfieri, Halle, Germany
David Hoza, Halle, Germany
Rights and permissions
About this article
Cite this article
Yilmaz, E.R., Kertmen, H., Gürer, B. et al. The protective effect of 2-mercaptoethane sulfonate (MESNA) against traumatic brain injury in rats. Acta Neurochir 155, 141–149 (2013). https://doi.org/10.1007/s00701-012-1501-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1501-3