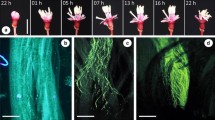
Abstract
Plant fitness strongly depends on the timing of flower production. In temperate climates most plants bloom during a relatively well-defined peak, while comparatively few species flower before or after the community peak. Since a phylogenetical signal has been shown to exist in the determination of reproductive phenology, it is of interest to identify characters associated with the emergence of either type of behaviour. Here we report on the reproduction of the late-flowering shrub Colletia hystrix and discuss the results in the context of the whole genus Colletia. Colletia hystrix shares with its congeners deep flowers, associated with assemblages of long-mouthpart pollinators, and characters that maximise the chances of successful pollen receipt and export in a single pollinator visit (homogamy, a large stigma, and an extragynoecial compitum). Leaflessness and extreme spinescence of Colletia are suggested to be related (via compromised resource acquisition) to phenological displacement and its flower-level correlates.

Similar content being viewed by others
References
Agrawal AA, Johnson MTJ, Hastings AP, Maron JL (2013) A field experiment demonstrating plant life-history evolution and its eco-evolutionary feedback to seed predator populations. Amer Naturalist 181:S35–S45
Aizen MA (2003) Influences of animal pollination and seed dispersal on winter flowering in a temperate mistletoe. Ecology 84:2613–2627
Aizen MA (2005) Breeding system of Tristerix corymbosus (Loranthaceae), a winter-flowering mistletoe from the southern Andes. Austral J Bot 53:357–361
Aizen MA, Ezcurra C (1998) High incidence of plant-animal mutualisms in the woody flora of the temperate forest of southern South America: biogeographical origin and present ecological significance. Ecol Austral 8:217–236
Aizen MA, Vázquez DP, Smith-Ramírez C (2002) Natural history and conservation of plant–animal mutualisms in the temperate forest of southern South America. Rev Chil Hist Nat 75:79–97
Aizen MA, Sabatino M, Tylianakis JM (2012) Specialization and rarity predict nonrandom loss of interactions from mutualist networks. Science 335:1486–1489
Arroyo MTK, Cavieres L, Peñaloza A, Riveros M, Faggi AM (1996) Relaciones fitogeográficas y patrones regionales de riqueza de especies en la flora del bosque lluvioso templado de Sudamérica. In: Armesto JJ, Villagrán C, Arroyo MTK (eds) Ecología de los bosques nativos de Chile. Editorial Universitaria, Santiago de Chile, pp 71–99
Basilio AM, Medan D (2001) Pollinator assemblages of Colletia spinosissima (Rhamnaceae): Composition, behavior, and specificity. Phyton (Buenos Aires) 2001:129–139
Basilio AM, Medan D, Torretta JP, Bartoloni NJ (2006) A year-long plant-pollinator network. Austral Ecol 31:975–983
Bertin IR, Newman CM (1993) Dichogamy in Angiosperms. Bot Rev 59:112–152
Dafni A, Motte Maués M (1998) A rapid and simple procedure to determine stigma receptivity. Sex Pl Reprod 11:177–180
D’Ambrogio A, Medan D (1993) Comportamiento reproductivo de Colletia paradoxa (Rhamnaceae). Darwiniana 32:1–14
Devoto M, Medan D, Montaldo NH (2005) Patterns of interaction between plants and pollinators along an environmental gradient. Oikos 109:461–472
Endress PK (1982) Syncarpy and alternative modes of escaping disadvantages of apocarpy in primitive Angiosperms. Taxon 31:48–52
Eskuche U (1999) Estudios fitosociológicos en el norte de la Patagonia II. Los bosques del Nothofagion dombeyi. Phytocoenologia 29:177–252
Fitzherbert W (1911) Colletia cruciata. Gard Chron 1911:255
Gallagher RV, Hughes L, Leishman MR (2009) Phenological trends among Australian alpine species: using herbarium records to identify climate-change indicators. Austral J Bot 57:1–9
Hinojosa LF, Villagrán C (1997) Historia de los bosques del sur de Sudamérica, I: antecedentes paleobotánicos, geológicos y climáticos del Terciario del cono sur de América. Rev Chil Hist Nat 70:225–539
Houston J, Hartley AJ (2003) The central andean west-slope rainshadow and its potential contribution to the origin of hyper-aridity in the Atacama desert. Int J Climatol 23:1453–1464
Johnson SD (1993) Climatic and phylogenetic determinants of flowering seasonality in the Cape flora. J Ecol 81:567–572
Kochmer JP, Handel SN (1986) Constraints and competition in the evolution of flowering phenology. Ecol Monog 56:303–325
Kudo G, Suzuki S (2002) Relationships between flowering phenology and fruit-set of dwarf shrubs in alpine fellfields in northern Japan: a comparison with a subarctic heathland in northern Sweden. Arctic Antarctic Alpine Res 34:185–190
Lavoie C, Lachance D (2006) A new herbarium-based method for reconstructing the phenology of plant species across large areas. Amer J Bot 93:512–516
Lindley J (1850) Memorandum concerning a remarkable case of vegetable transformation. J Hort Soc London 5:29–32
Lloyd DG, Webb CJ (1986) The avoidance of interference between the presentation of pollen and stigmas in Angiosperms I. Dichogamy. New Zealand J Bot 24:135–162
Mahoro S (2003) Effects of flower and seed predators and pollinators on fruit production in two sequentially flowering congeners. Pl Ecol 166:37–48
Markgraf V, McGlone M, Hope G (1995) Neogene paleoenvironmental and paleoclimatic change in southern temperate ecosystems: a southern perspective. Trends Ecol Evol 10:143–147
Medan D (1991) Reproductive phenology, pollination biology, and gynoecium development in Discaria americana (Rhamnaceae). New Zealand J Bot 29:31–42
Medan D (2003) Reproductive biology of the Andean shrub Discaria nana (Rhamnaceae). Pl Biol 5:94–102
Medan D, Aagesen L (1995) Comparative flower and fruit structure in the Colletieae (Rhamnaceae). Bot Jahrb Syst 117:531–564
Medan D, Arce ME (1999) Reproductive biology of the Andean-disjunct genus Retanilla (Rhamnaceae). Pl Syst Evol 218:281–298
Medan D, Basilio AM (2001) Reproductive biology of Colletia spinosissima (Rhamnaceae) in Argentina. Pl Syst Evol 229:79–89
Medan D, D’Ambrogio AC (1998) Reproductive biology of the andromonoecious shrub Trevoa quinquenervia (Rhamnaceae). Bot J Linn Soc 126:191–206
Medan D, Devoto M (2005) Reproductive ecology of a perennial outcrosser with a naturally dissected distribution. Pl Syst Evol 254:173–184
Medan D, Montaldo NH (2005) Ornithophily in the Rhamnaceae: the pollination of the Chilean endemic Colletia ulicina. Flora 200:339–344
Medan D, Basilio AM, Devoto M, Bartoloni NJ, Torretta JP, Petanidou T (2006) Measuring generalization and connectance in temperate, long-lasting systems. In: Waser N, Ollerton J (eds) Plant-pollinator interactions. From specialization to generalization. University of Chicago, Chicago, pp 245–259
Medan D, Zarlavsky G, Bartoloni NJ (2013) Plant reproduction in the high-Andean Puna: Kentrothamnus weddellianus (Rhamnaceae: Colletieae). Pl Syst Evol 299:841–851
Morales MA, Dodge GJ, Inouye DW (2005) A phenological mid-domain effect in flowering diversity. Oecologia 142:83–89
Morales CL, Arbetman MP, Cameron SA, Aizen MA (2013) Rapid ecological replacement of a native bumble bee by invasive species. Front Ecol Environ. doi:10.1890/120321
Movia CP, Ower GH, Pérez CE (1982) Estudio de la vegetación natural de la Provincia del Neuquén. Ministerio de Economía y Hacienda. Subsecretaría de Estado de Recursos Naturales, Neuquén (Argentina)
Munguía-Rosas MA, Ollerton J, Parra-Tabla V, De-Nova JA (2011) Meta-analysis of phenotypic selection on flowering phenology suggests that early flowering plants are favoured. Ecol Lett. doi:10.1111/j.1461-0248.2011.01601.x
Paruelo JM, Beltrán AM, Jobbágy E et al (1998) The climate of Patagonia: general patterns and controls on biotic processes. Ecol Austral 8:85–101
Pilson D (2000) Herbivory and natural selection on flowering phenology in wild sunflower, Helianthus annuus. Oecologia 122:72–82
Primack RB (1979) Reproductive biology of Discaria toumatou (Rhamnaceae). New Zealand J Bot 17:9–13
Riveros MG, Smith-Ramírez C (1996) Patrones de floración y fructificación en bosques del sur de Chile. In: Armesto J, Villagrán C, Arroyo MTK (eds) Ecología de los bosques nativos de Chile. Editorial Universitaria, Santiago, pp 71–100
Robbirt KM, Davy AJ, Hutchings MJ, Roberts DL (2011) Validation of biological collections as a source of phenological data for use in climate change studies: a case study with the orchid Ophrys sphegodes. J Ecol 99:235–241
Roig FA (1998) La vegetación de la Patagonia. In: Correa MN (ed) Flora Patagónica, vol 1. INTA, Buenos Aires, pp 48–174
Ronel M, Ne’eman G, Lev-yadun S (2010) Spiny east Mediterranean plant species flower later and in a drier season than non-spiny species. Flora 205:276–281
Skottsberg C (1928) Pollinationsbiologie und Samenverbreitung auf den Juan Fernandez-Inseln. In: Skottsberg C (ed) The natural history of Juan Fernandez and Easter Island, vol 2. Almqvist & Wiksell, Uppsala, pp 503–547
Sola AJ, Ehrlén J (2007) Vegetative phenology constrains the onset of flowering in the perennial herb Lathyrus vernus. J Ecol 95:208–216
Thiers B (2014, continuously updated) Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/
Torretta JP, Medan D, Abrahamovich AA (2006) First record of the invasive bumblebee Bombus terrestris (L.) (Hymenoptera, Apidae) in Argentina. Trans Amer Entomol Soc 132:285–289
Tortosa RD (1989) El género Colletia (Rhamnaceae). Parodiana 5:279–332
Tortosa RD, Medan D (1989) Novedades sobre nódulos actinomicorrícicos en angiospermas sudamericanas. Rev Fac Agronomía UBA 10:79–86
Tortosa RD, Aagesen L, Tourn GM (1996) Morphological studies in the tribe Colletieae (Rhamnaceae): analysis of architecture and inflorescences. Bot J Linn Soc 122:353–367
Valtueña FJ, Ortega-Olivencia A, Rodríguez-Riaño T, López J (2008) Reproductive biology in Anagyris foetida L. (Leguminosae), an autumn-winter flowering and ornithophilous Mediterranean shrub. Bot J Linn Soc 157:519–532
Villagrán C, Hinojosa LF (1997) Historia de los bosques del sur de Sudamérica II: Análisis fitogeográfico. Rev Chil Hist Nat 70:241–267
Acknowledgments
R. González-Vaquero (Museo Argentino de Ciencias Naturales, Buenos Aires) and F. Navarro (Instituto Miguel Lillo, Tucumán) helped with insect identification. M.C. Alvarez, G. Zarlavsky, A.D’Ambrogio and S. Fachino helped in the field and laboratory, and N.J. Bartoloni assisted with statistical analyses. A. Premoli and J. Kellermann provided bibliographic sources. C. Morales and M. Sabatino offered useful insights. Comments by M. Devoto, N.H. Montaldo and an anonymous reviewer helped to improve a previous draft. Financial support was granted by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and Universidad de Buenos Aires. DM and JPT are affiliated with CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medan, D., Torretta, J.P. The reproduction of Colletia hystrix and late-flowering in Colletia (Rhamnaceae: Colletieae). Plant Syst Evol 301, 1181–1189 (2015). https://doi.org/10.1007/s00606-014-1142-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1142-5