Abstract
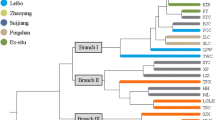
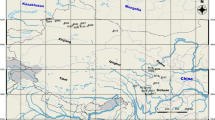
Anemone shikokiana (Makino) Makino, which is distributed only in Mt. Kunyu and Mt. Lao of Shandong Province in China and Mt. Shichui of Shikoku in Japan above 600 m in elevation, is a rare plant. This species has two obviously different habitats, the shrubs in mountain top and understory of the conifer and broadleaf mixed forest. In this study, the habitats and genetic diversity were analyzed for exploring the effects of habitats on the genetic diversity of A. shikokiana. Hanfengling and Taiboding of Mt. Kunyu, Hualiukou and Laoding of Mt. Lao were chosen as four representative plots for analysis and sampling. The levels of genetic diversity and population structure of four populations were examined using amplified fragment length polymorphism markers. Six primer combinations produced a total of 474 replicated bands, of which 435 (91.77 %) were polymorphic among populations. The percentage of polymorphic bands within populations ranged from 62.45 to 68.14 %. The results were as follows: the genetic diversity among populations was much higher than that within populations; the genetic diversity in Mt. Kunyu was higher than that in Mt. Lao, the genetic diversity in the understory of conifer and broadleaf mixed forest was higher than it in shrubs of mountaintop. We think that the genetic diversity is related with geographic distance and habitats. The result of clustering is not fully in accordance with their geographical distributions, but in accordance with habitats. Based on our study, we concluded that this weed species potentially has broad adaptability, its genetic diversity is correlated to geographic distance and habitats, and attention should be paid to preserve every population and their habitats.



Similar content being viewed by others
References
Booy G, Hendriks RJJ, Smulders MJM et al (2000) Genetic diversity and the survival of populations. Plant Biol 2:379–395
Brunet J, Oheimb VG (1998) Colonization of secondary woodlands by Anemone nemorosa. Nord J Bot 18:369–377
Brütting C, Meyer S, Kühne P et al (2012) Spatial genetic structure and low diversity of the rare arable plant Bupleurum rotundifolium L. indicate fragmentation in Central Europe. Agr Ecosyst Environ 161:70–77
Chen YY, Li l, Wei L (2010) Genetic diversity and population structure of the endangered alpine quillwort Isoetes hypsophila Hand.-Mazz. Revealed by AFLP markers. Plant Syst Evol 290:127–139
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Excoffier L (1995) AMOVA 1.55 (analysis of molecular variance). University of Geneva, Geneva
Frankham R (2010) Challenges and opportunities of genetic approaches to biological conservation. Biol Conserv 143:1919–1927
Gaudeul M, Taberlet P, Till-Bottraud I (2000) Genetic diversity in an endangered alpine plant, Eryngium alpinum L. (Apiaceae), inferred from amplified fragment length polymorphism markers. Mol Ecol 9:1625–1637
Hafdís HÆ, Patrick K, Jürg S (2009) Isolated populations of a rare alpine plant show high genetic diversity and considerable population differentiation. Ann Bot 104:1313–1322
Hamrick JL, Godt MJW (1989) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics breeding and genetic resources. Sinauer Associates, Sunderland, pp 43–63
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos T R Soc B 351:1291–1298
Helen B, Sarah B, Nina OT et al (2013) Identifying genetic signatures of selection in a non-model species, alpine gentian (Gentiana nivalis L.), using a landscape genetic approach. Conserv Genet 14:467–481
Holderegger R (1996) Effects of litter removal on the germination of Anemone nemorosa L. Flora 191:175–177
Huang Y, Zhang CQ, Li DZ (2009) Low genetic diversity and high genetic differentiation in the critically endangered Omphalogramma souliei (Primulaceae): implications for its conservation. J Syst Evol 47:103–109
Jennerston O (1988) Pollination in Dianthus deltoides(Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conserv Biol 2:359–366
Ji H, He CZ (2010) Population structure and genetic diversity of Huperzia serrata (Huperziaceae) based on amplified fragment length polymorphism (AFLP) markers. Biochem Syst Ecol 38:1137–1147
Laikre L (2010) Genetic diversity is overlooked in international conservation policy implementation. Conserv Genet 11:349–354
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure in plant populations. Annu Rev Ecol Syst 15:65–95
Nei M (1972) Genetic distance between populations. Am Nat 106:283–392
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 79:3321–3323
Nei M (1987) Molecular evolution genetics. Columbia University Press, New York
Nybom H, Bartish I (2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect Plant Ecol Evol Syst 3:93–114
Ouborg NJ, Vergeer P, Mix C (2006) The rough edges of the conservation genetics paradigm for plants. J Ecol 94:1233–1248
Pickup M, Young AG (2008) Population size, self-incompatibility and genetic rescue in diploid and tetraploid races of Rutidosis leptorrhynchoides (Asteraceae). Heredity 100:268–274
Sen N, Tsuyoshi Y, Shinji F et al (2008) Spatial patterns and habitat associations of Fagaceae in a hill dipterocarp forest in Ulu Gadut, West Sumatra. J Trop Ecol 24:535–550
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Shirrefs DA (1985) Biological Flora of the British Isles: Anemone nemorosa L. J Ecol 73:1005–1020
Slatkin M (1987) Gene flow and geographic structure of natural populations. Science 236:787–792
Slatkin M, Barton NH (1989) A comparison of three indirect methods for estimating average levels of gene flow. Evolution 43:1349–1368
Spielman D, Brook BW, Frankham R (2004) Most species are not driven to extinction before genetic factors impact them. Proc Natl Acad Sci USA 101:15261–15264
Stehlik I, Holderegger R (2000) Spatial genetic structure and clonal diversity of Anemone nemorosa in late successional deciduous woodlands of Central Europe. J Ecol 88:424–435
Theurillat JP, Guisan A (2001) Potential impact of climate change on vegetation in the European Alps: a review. Clim Change 50:77–109
Vergeer P, Rengelink R, Copal A, Ouborg NJ (2003) The interacting effects of genetic variation, habitat quality and population size on the performance of succisa pratensis. J Ecol 91:18–26
Vos P, Hogers R, Bleeker M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wang ZJ, Guan KY (2011) High genetic diversity and low genetic differentiation in the relict tree fern Sphaeropteris brunoniana (Cyatheaceae) revealed by amplified fragment length polymorphism (AFLP). Bot Stud 52:231–238
Wang Z, Pang YJ, Liu CL, Zhuang SH, Bian FH (2013) Karyotype analysis and influence of habitats on chromosomal variation of rare plant Anemone shikokiana (Makino) Makino. Bot Res 2:113–116
Wang Z, Pang YJ, Liu CL, Bian FH (2014) Distribution pattern of rare plant Anemone shikokiana (Makino) makino and analysis of influencing factors. Bull Bot Res 34:440–445
Webb CO, Peart DR (2000) Habitat associations of trees and seedlings in a Bornean rain forest. J Ecol 88:464–478
Xu W, Bai WR, Wen GJ et al (2013) Influences of harvesting on genetic diversity and population structure of Anemone altaica (Ranunculaceae), a traditional Chinese medicinal herb. Biochem Syst Ecol 47:121–125
Young AG, Merriam HG, Warwick SL (1993) The effects of forest fragmentation on genetic variation in Acer saccbarum Marsh. (Sugar maple) populations. Heredity 71:277–289
Young AG, Boyle T, Brown AHD (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Zhang FM, Song G (2002) Data analysis in population genetics.I.analysis of RAPD data with AMOVA. Biodiversity Sci 10:438–444
Zheng W, Wang L, Meng L, Liu J (2008) Genetic variation in the endangered Anisodus Tanguticus (Solanaceae), an alpine perennial endemic to the Qinghai-Tibetan Plateau. Genetica 132:123–129
Acknowledgments
We would like to thank Mr. Chen Peng and Mr. Deng Jianping for collecting the plant materials of A. shikokiana. This work was financially sponsored by grants from the National Natural Science Foundation of China (31100395).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bian, F., Pang, Y., Wang, Z. et al. Genetic diversity of the rare plant Anemone shikokiana (Makino) Makino (Ranunculaceae) inferred from AFLP markers. Plant Syst Evol 301, 677–684 (2015). https://doi.org/10.1007/s00606-014-1105-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1105-x