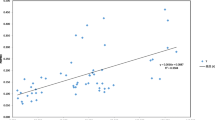
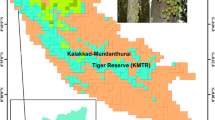
Abstract
Coscinium fenestratum is a critically endangered medicinal plant, well-known for its bioactive isoquinoline alkaloid berberine. The species has been over harvested from its natural habitats to meet the huge requirement of raw drug market and industrial consumption. This has lead to a rapid decline in the population size and has also led to local population extinction at few locations in the Western Ghats, India. In this study, inter-simple sequence repeat markers were used to investigate the genetic variation and population structure of seven extant populations of C. fenestratum from the central Western Ghats, India. Eight primer combination produced a total of 57 unambiguous bands, of which (47.1 %) were polymorphic. The species exhibited a moderate to low level of intra population genetic diversity (H s = 0.347 ± 0.008; H t = 0.378 ± 0.006 (POPGENE) and H s = 0.262 ± 0.0028; H t = 0.204 ± 0.020 (HICKORY)). The populations were low to moderately differentiated from one another (G ST = 0.221) and geographical distance was not significantly correlated with genetic distance, suggesting that these long-lived, geographically distant remnant populations were once connected through gene flow. There was a significant amount of genetic variation among populations (19.85 %). The Bayesian software STRUCTURE and HICKORY were used to further reveal the genetic structure of C. fenestratum. The results revealed weak population structure (K = 2) with one single widespread gene pool, and indicated that gene flow and inbreeding are likely to be the major driving force in shaping current population genetic structure of C. fenestratum. Thus, an understanding of the genetic diversity and population structure of C. fenestratum can provide insight into the conservation and management of this species.



Similar content being viewed by others
References
Abdul Kareem VK, Rajasekharan PE, Mini S, Vasantha Kumar T (2011) Genetic diversity and structure of the threatened anti-cancerous plant Nothapodytes nimmoniana as revealed by ISSR analysis. Plant Genet Resour 9(4):506–514
Agusta A (2003) Coscinium fenestratum (Gaertner) Colebr. In: Lemens RMHJ, Bunyapraphatsara N (eds) Plant resources South East Asia: medicinal and poisonous plants, vol 3. PROSEA, Bogor, pp 139–140
Ahmad B (1998) Plant exploration and documentation in view of land clearing in Sabah. In: Nair MNB, Ganapathi N (eds) Medicinal plants. Cure for the 21st century, biodiversity conservation and utilization of medicinal plants. Universiti Putra Malaysia, Serdang, pp 161–162
Amri E, Mamboya F (2012) Genetic diversity in Pterocarpus angolensis detected by random amplified polymorphic DNA markers. Int J Plant Breed Genet 6(2):105–114
Astorga JG, Campos CG (2004) Genetic variability of the narrow endemic tree Antirhea aromatica (Rubiaceae, Guettardeae) in a tropical forest of Mexico. Ann Bot 93:521–528
Birdsall TCND, Kelly SND (1997) Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Alt med Rev 2:94–103
Canter PH (2005) Bringing medicinal plants into cultivation. Focus Altern Compl Ther 10:167–168
Cunningham AB (1993) African medicinal plants. Setting priorities at the interface between conservation and primary healthcare. People and plants working paper 1. UNESCO, Paris
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Earl DA, von Holdt BM (2009) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Cons Genet Res 4:359–361
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Ann Rev Ecol Syst 24:217–242
Evanno G, Regnaut S, Goudet J (2007) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620
Fang DQ, Roose ML, Krueger RR, Federici CT (1997) Fingerprinting trifoliate orange germplasm accessions with isozymes, RFLPs and inter-simple sequence repeat markers. Theor Appl Genet 95:211–219
Ferreira ME, Grattapaglia D (1995) Introdução ao uso de marcadores moleculares em análise genética. EMBRAPA-CENARGEN, Brasília
Fischer M, Matteis D (1998) Effects of population size on performance in the rare plant Gentianella germanica. J Ecol 86:195–204
Francisco-Ortega J, Santos-Guerra A, Kim SC, Crawford DJ (2000) Plant genetic diversity in the Canary Islands: a conservation perspective. Am J Bot 87:909–919
Frankel H, Soule ME (1981) Conservation and evolution. Cambridge University Press, New York
Frankham R (1995) Inbreeding and extinction: a threshold effect. Conserv Biol 9:792–799
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge, p 617
Ganeshaiah KN (2003) Sasya Sahyadri: distribution, taxonomy and diversity of plants of Western Ghats. University of Agricultural Sciences, Bangalore
Godt MJ, Hamrick JL (2001) Genetic diversity in rare Southeastern plants. Nat Area J 21:61–70
Godt MJ, Hamrick JL, Bratton S (1995) Genetic diversity in a threatened wetland species, Helonias bullata (Liliaceae). Conserv Biol 9:596–604
Hamilton AC (1997) Threats to plants: an analysis of centers of plant diversity. In: Touchell DH, Dixon KW (eds) Conservation into the 21st Century. Proceedings of 4th International Botanic Gardens Conservation Congress, Perth, pp 309–322
Hamrick JL, Godt MJ (1989) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS (eds) Plant population genetics, breeding, and genetic resources. Sinauer Associates, Sunderland, pp 43–63
Holsinger KE, Lewis PO (2003) HICKORY: a package for analysis of population genetic data, Version 8_0. University of Connecticut, Storrs
Holsinger KE, Wallace LE (2004) Bayesian approaches for the analysis of population genetic structure: an example from Platanthera leucophaea (Orchidaceae). Mol Ecol 13:887–894
Holsinger KE, Lewis PO, Dey DK (2002) A Bayesian approach to inferring population structure from dominant markers. Mol Ecol 11:1157–1164
Hu Y, Wang L, Xie X, Yang J, Li Y, Zhang H (2010) Genetic diversity of wild populations of Rheum tanguticum endemic to China as revealed by ISSR analysis. Biochem Syst Ecol 38:264–274
Hutchison DW, Templeton AR (1999) Correlation of pairwise genetic and geographic distance measures: inferring the relative influence of gene flow and drift on the distribution of genetic variability. Evolution 53:1898–1914
Jakobsson M, Rosenberg NA (2007) CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806
Jiang Y, Li Y, Lu S, Liu Y, Peng J, Zhu D (2012) Genetic diversity of Chimonanthus grammatus populations determined with inter-simple sequence repeats (ISSR) analysis: implications for conservation. J Med Plant Res 6(7):1272–1278
Jin Z, Li J (2007) Genetic differentiation in endangered Heptacodium miconioides Rehd. based on ISSR polymorphism and implications for its conservation. For Ecol Manag 245:130–136
Kala CP (2005) Indigenous uses, population density, and conservation of threatened medicinal plants in protected areas of the Indian Himalayas. Conserv Biol 19:368–378
Kareem AVK, Rajasekharan PE, Mini S, Vasantha Kumar T (2012) Genetic diversity and structure of the threatened anticancerous plant Nothapodytes nimmoniana as revealed by ISSR analysis. Plant Genet Resour: Charact Util 9(4):506–514
Keiper FJ, McConchie R (2000) An analysis of genetic variation in natural populations of Sticherus flabellatus [R. Br. (St John)] using amplified fragment length polymorphism (AFLP) markers. Mol Ecol 9:571–581
Kolammal M (1978) Pharmacognosy of Ayurvedic Drugs. Series I. No. 2. Department of Pharmacology, Government Ayurveda College, Thiruvananthapuram
Kothera L, Richards CM, Carney SE (2007) Genetic diversity and structure in the rare Colorado endemic plant Pisarı′a bellii (Brassicaceae). Conserv Genet 8:1043–1050
Krauss SL (2000) Accurate gene diversity estimates from amplified length polymorphism (AFLP) markers. Mol Ecol 9:1241–1245
Lande R (1995) Mutation and conservation. Conserv Biol 9:782–791
Lange D (1998) Europe’s medicinal and aromatic plants: their use, trade and conservation: an overview. TRAFFIC International, Cambridge
Laurance WF (1999) Reflections on the tropical deforestation crisis. Biol Coserv 91:109–117
Lewis WH (1988) Re-growth of a decimated Population of (Pana quinquefolium) in a Missouri climax forest. Rhodora 90:1–5
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Mahar KS, Rana TS, Ranade SA, Pande V, Palni LMK (2013) Estimation of genetic variability and population structure in Sapindus trifoliatus L., using DNA fingerprinting methods. Trees 27(1):85–96
Menges ES (1991) The application of minimum viable population theory to plants. In: Falk DA, Holsinger KE (eds) Genetics and the conservation of rare plants, Centre for Plant Conservation. Oxford University Press, New York, pp 45–61
Mills LS, Smouse PE (1994) Demographic consequences of inbreeding in remnant populations. Am Nat 144:412–431
Mittermeier RA, Robles GP, Hoffman M, Piligrim JD, Brooks TM, Mittermeier CG, Lamoreux JL, Fonseca G (2004) Hotspots revisited: earths biologically richest and most endangered terrestrial ecoregions. Cemex, Mexico
Mohanan N, Sivadasan M (2002) Flora of Agasthyamala. Bishen Singh Mahendra Pal Singh, Dehradun, p 65
Nagaoka T, Ogihara Y (1997) Applicability of inter-simple sequence repeat polymorphisms in wheat for use asDNA markers in comparison to RFLP and RAPD markers. Theor Appl Genet 94:597–602
Nagaraju J, Reddy K, Nagaraja G, Sethuraman B (2001) Comparison of multilocus RFLPs and PCR-based marker systems for genetic analysis of the silk-worm, Bombix mori. Heredity 86:588–597
Nambiar VPK, Warrier PK, Gangapathy PM (2000) Some important medicinal plants of the Western Ghats, India. A profile. AVS publication, IDRC, Artstock, New Dehli, pp 105–120
Nantel P, Gagnon D, Nault A (1996) Population viability analysis of American ginseng and wild leek harvested in stochastic environments. Conserv Biol 10:608–621
Narasimhan S, Padmesh P, Nair GM (2006) Assessment of genetic diversity in Coscinium fenestratum. Biol Plant 50(1):111–113
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Newman D, Pilson D (1997) Increased probability of extinction due to decreased genetic effective population size: experimental populations of Clarkia pulchella. Evolution 51:354–362
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Padmesh P, Sabu KK, Seeni S, Pushpangadan P (1999) The use of RAPD in assessing genetic variability in Andrographis paniculata Nees, a hepatoprotective drug. Curr Sci 76:833–835
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research molecular ecology notes, http://www.anu.edu.au/BoZo/GenAlEx/, 6: 288–295
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Ramasubbu R, Prabha AC, Kumuthakalavalli R (2012) Seed biology of Coscinium fenestratum (Gaertn.) Colebr.-a critically endangered medicinal plant of Western Ghats. J Med Plant Res 6(6):1094–1096
Ravikanth G, Nageswara Rao M, Ganeshaiah KN, Uma Shaanker R (2009) Genetic diversity of NTFP species: issues and implications. In: Uma Shaanker R, Hiremath AJ, Joseph GC, Rai N (eds) Non-timber forest products conservation, management and policies. Ashoka Trust for Research in Ecology and Environment, Bangalore and Forestry Research Support Program for Asia and the Pacific, Food and Agriculture Organisation, Bangkok, pp 53–64
Ravikumar K, Ved DK (2000) 100 red listed medicinal plants of conservation concern in South India. FRLHT, Bangalore, pp 99–103
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Robbins CS (1998) American ginseng: the root of North America’s medicinal herb trade. TRAFFIC North America Report. Number B347, Washington
Rossetto M, Weaver PK, Dixon KW (1995) Use of RAPD analysis in devising conservation strategies for the rare and endangered Grevillea scapigera (Proteaceae). Mol Ecol 4:321–329
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Schneider S, Roessli D, Excoffier L (2000) Arlequin: a software for population genetic data: genetics and biometry laboratory. University of Geneva, http://lgb.unige.ch/arlequin/
Sehgal R (2012) Indian medicinal plant face extinction. The Asian age. http://www.asianage.com/india/Indian-medicinal-plants-face-extinction-771. Accessed 5 Jan 2013
Shah A, Li DZ, Gao LM, Li HT, Möller M (2008) Genetic diversity within and among populations of the endangered species Taxus fuana (Taxaceae) from Pakistan and implication of its conservation. Biochem Syst Ecol 36:183–193
Shannon CE, Weaver W (1949) The mathematical theory of communication. Univ. Illinois Press, London and New York
Sheldon JW, Balick MJ, Laird (1997) Medicinal plants: can utilization and conservation coexist? Advances in economic botany, vol 12. Bronx, New York
Shilpha J, Silambarasan T, Pandian SK, Ramesh M (2013) Assessment of genetic diversity in Solanum trilobatum L., an important medicinal plant from South India using RAPD and ISSR markers. Genetic Resource and crop. Evolution 60(3):807–818
Slatkin M (1977) Gene flow and genetic drift in a species subject to frequent local extinctions. Theor Popul Biol 12:253–262
Su YJ, Zan QJ, Wang T, Ying ZM, Ye HG (2008) High ISSR variation in 24 surviving individuals of Apterosperma oblata (Theaceae) endemic to China. Biochem Syst Ecol 36:619–625
Sumy O, Ved DK, Krishnan R (2000) Tropical Indian medicinal plants: propagation methods. FRLHT, Bangalore, pp 114–115
Tandon V (1996) CAMP workshop–plants under threat new list forged. Med Plants Conserv Newsl 2:12–13
Tran TA, Ziegler S (2001) Utilization of medicinal plants in Bach Ma National Park, Vietnam. Newsletter Med. Plant Specialist Group IUCN Species. Surv Comm 7:3–4
Tushar KV, Udayan PS (2005) Ex situ conservation of ayurvedic medicinal plants at Arya vaidya sala, Kottakkal. In: Proceedings of XVIIth Kerala Science Congress, Jan 29–31, Kerala Forest Research Institute (KFRI) Peechi, Thrissur, p 311
Uma Shaanker R, Ganeshaiah KN, Nageswara Rao M, Ravikanth G (2002) Forest gene banks—a new integrated approach for the conservation of forest tree genetic resources. In: Engels JMM, Brown AHD, Jackson MT (eds) Managing plant genetic resources. CABI Publishing, Wallingford, pp 229–235
Ved DK, Mudappa A, Shanker D (1998) Regulating export of endangered medicinal plants species need for scientific region. Curr Sci 75:341–344
Vrijenhoek RC, Douglas ME, Meffe GK (1985) Conservation genetics of endangered fish populations in Arizona. Science 229:400–402
Wright S (1943) Isolation by distance. Genetics 28:139–156
Yeh FC, Yang RC, Boyle T (1999) POPGENE, version 1.31. Microsoft windows based freeware for population genetic analysis. http://www.ualberta.ca/fyeh/fyeh
Zhivotovsky LA (1999) Estimating population structure in diploid with multilocus dominant DNA markers. Mol Ecol 8:907–913
Zong M, Liu HL, Qiu YX, Yang SZ, Zhao MS, Fu CX (2008) Genetic diversity and geographic differentiation in the threatened species Dysosma pleiantha in China as revealed by ISSR analysis. Biochem Genet 46:108–196
Acknowledgments
We would like to thank Karnataka Forest Department for the permission to collect the samples in the protected area and for providing the species distribution data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thriveni, H.N., Sumangala, R.C., Shivaprakash, K.N. et al. Genetic structure and diversity of Coscinium fenestratum: a critically endangered liana of Western Ghats, India. Plant Syst Evol 300, 403–413 (2014). https://doi.org/10.1007/s00606-013-0890-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0890-y