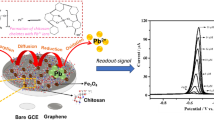
Abstract
A hybrid conjugate of reduced graphene oxide/ferrous-ferric oxide nanoparticles (rGO-Fe3O4 NPs) is characterized and assembled with chitosan and laccase to form a layered functional superstructure. After its characterization by field-effect scanning electron microscopy, energy-dispersive X-ray analysis, X-ray photoelectron spectroscopy, attenuated total reflectance Fourier transform infrared, cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS), the nanocomposite has been deposited on glassy carbon for the enzyme-mediated electrochemical determination of the endocrine disruptor bisphenol A (BPA). Proof-of-concept assays conducted by using CV, EIS, and square wave voltammetry reveal that the enzymatic biosensor provides linear response in a wide range of BPA concentrations (6–228 ppb), very high sensitivities, and excellent durability (over 1-month storage). Using amperometric detection, remarkable sensitivities (2080 μA μM−1 cm−2) and detection limits (18 nM) are attained. Applications to real samples of bottled water proved feasible with recoveries in the range 107–124%.

Reduced graphene oxide conjugated with magnetite nanoparticles (rGO-Fe3O4) was assembled with laccase (wine-colored dots) and chitosan for the electrochemical determination of bisphenol A. The enzymatic biosensor exhibited excellent linearity (6–228 ppb) and stability. Best sensitivity (2080 μA μM−1 cm−2, detection limit 18 nM) was obtained by amperometry.




Similar content being viewed by others
References
Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S-I, Kimura M, Shimohigashi Y (2007) Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERRγ. J Biochem 142:517–524
Vom Saal FS, Hughes C (2005) An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113:926–933
Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS (2010) In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Canc 1:146–155
Tarapore P, Ying J, Ouyang B, Burke B, Bracken B, Ho SM (2014) Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS One 9(3):e90332
Cao XL, Corriveau J (2008) Migration of bisphenol A from polycarbonate baby and water bottles under severe conditions. J Agric Food Chem 56:6378–6381
Nerín C, Philo MR, Salafranca J, Castle L (2002) Determination of bisphenol-type contaminants from food packaging materials in aqueous foods by solid-phase microextraction-high-performance liquid chromatography. J Chromatogr A 963:375–980
Brenn-Struckhofova Z, Cichna-Markl M (2006) Determination of bisphenol A in wine by sol-gel immunoaffinity chromatography, HPLC, and fluorescence detection. Food Addit Contam 23:1227–1235
Yang Y, Asiri AM, Tang Z, Du D, Lin Y (2013) Graphene based materials for biomedical applications. Mater Today 16:365–373
Dalfovo MC, Lacconi GI, Moreno M, Yappert MC, Sumanasekera GU, Salvarezza RB, Ibañez FJ (2014) Synergy between graphene and Au nanoparticles (Heterojunction) towards quenching, improving Raman signal, and UV light sensing. ACS Appl Mater Interfaces 6:6384–6391
Su B, Shao H, Li N, Chen X, Cai Z, Chen X (2017) A sensitive bisphenol A voltammetric sensor relying on AuPd nanoparticles/graphene composites modified glassy carbon electrode. Talanta 166:126–132
Krishna R, Campiña JM, Fernandes PMV, Ventura J, Titus E, Silva AF (2016) Reduced graphene oxide-nickel nanoparticles/biopolymer composite films for the sub-millimolar detection of glucose. Analyst 141:4151–4161
Albelda JAV, Uzunoglu A, Santos GNC, Stanciu LA (2017) Graphene-titanium dioxide nanocomposite based hypxanthine sensor for assessment of meat freshness. Biosens Bioelectron 89:518–524
Zhang Y, Cheng Y, Zhou Y, Li B, Gu W, Shi X, Xian Y (2013) Electrochemical sensor for bisphenol A based on magnetic nanoparticles decorated reduced graphene oxide. Talanta 107:211–218
Yu C, Gou L, Zhou X, Bao N, Gu H (2011) Chitosan-Fe3O4 nanocomposite based electrochemical sensors for the determination of bisphenol A. Electrochim Acta 56:9056–9063
Han J, Li F, Jiang L, Li K, Dong Y, Li Y (2015) Electrochemical determination of bisphenol A using a polyacrylamide-multiwalled carbon nanotube-modified glassy carbon electrode. Anal Methods 7:8220–8226
Qin J, Shen J, Xu X, Yuan Y, He G, Chen H (2018) A glassy carbon electrode modified with nitrogen-doped reduced graphene oxide and melamine for ultra-sensitive voltammetric determination of bisphenol A. Microchim Acta 185:459
Mirzajani H, Cheng C, Wu J, Chen J, Eda S, Aghdam EN, Ghavifekr HB (2017) A highly sensitive and specific capacitive aptasensors for rapid and label-free trace analysis of bisphenol A (BPA) in canned foods. Biosens Bioelectron 89:1059–1067
Ensafi AA, Amini M, Rezaei B (2018) Molecularly imprinted electrochemical aptasensor for the attomolar detection of bisphenol A. Microchim Acta 185:265
Baghayeri M, Ansari R, Nodehi M, Razavipanah I, Veisi H (2018) Voltammetric aptasensor for bisphenol A based on the use of a MWCNT/Fe3O4@gold nanocomposite. Microchim Acta 185:320
Kim KS, Jang JR, Choe WS, Yoo PJ (2015) Electrochemical detection of bisphenol A with high sensitivity and selectivity using recombinant protein-immobilized graphene electrodes. Biosens Bioelectron 71:214–221
Kim SG, Lee JS, Jun J, Shin DH, Jang J (2016) Ultrasensitive bisphenol A field-effect transistor sensor using an aptamer-modified multichannel carbon nanofiber transducer. ACS Appl Mater Interfaces 8(10):6602–6610
Zhu Y, Cai Y, Xu L, Zheng L, Wang L, Qi B, Xu C (2015) Building an aptamer/graphene oxide FRET biosensor for one-step detection of bisphenol A. ACS Appl Mater Interfaces 7(14):7492–7496
Cajthaml T, Kresinova Z, Svobodova K, Moder M (2009) Biodegradation of endocrine-disrupting compounds and suppression of estrogenic activity by ligninolytic fungi. Chemosphere 75:745–750
Abdullah J, Ahmad M, Heng LY, Karuppiah N, Sidek H (2006) Immobilization of tyrosinase in chitosan film for an optical detection of phenol. Sensors Actuators B Chem 114:604–609
Abdullah J, Ahmad M, Heng LY, Karuppiah N, Sidek H (2006) Stacked films immobilization of MBTH in nafion/sol-gel silicate and horseradish peroxidase in chitosan for the determination of phenolic compounds. Anal Bioanal Chem 386:1285–1292
Rodríguez-Delgado MM, Alemán-Nava GS, Rodríguez-Delgado JM, Dieck-Assad G, Martínez-Chapa SO, Barceló D, Parra R (2015) Laccase-based biosensors for detection of phenolic compounds. TrAC-Trend Anal Chem 74:21–45
Freire RS, Duran N, Kubota LT (2002) Development of a laccase-based flow injection electrochemical biosensor for the determination of phenolic compounds and its application for monitoring remediation of Kraft E1 paper mill effluent. Anal Chim Acta 463:229–238
Casero E, Petit-Domínguez MD, Vázquez L, Ramírez-Asperilla I, Parra-Alfambra AM, Pariente F, Lorenzo E (2013) Laccase biosensors based on different enzyme immobilization strategies for phenolic compounds determination. Talanta 115:401–408
Portaccio M, Di Tuoro D, Arduini F, Moscone D, Cammarota M, Mita DG, Lepore M (2013) Laccase biosensor based on screen-printed electrode modified with thionine-carbon black nanocomposite for bisphenol A detection. Electrochim Acta 109:340–347
Lou C, Jing T, Tian J, Zheng Y, Zhang J, Dong M, Wang C, Hou C, Fan J, Guo Z (2019) 3-Dimensional graphene/Cu/Fe3O4 composites: immobilized laccase electrodes for detecting bisphenol A. J Mater Res 34(17):2964–2975
Dyshlyuk LS, Novoselova MV, Rozalenok TA (2013) Immobilization of chymotrypsin on magnetic Fe3O4 nanoparticles. Foods Raw Mater 1:85–88
Zhao G, Wang J, Li Y, Chen X, Liu Y (2011) Enzymes immobilized on superparamagnetic Fe3O4@clays nanocomposites: preparation, characterization, and new strategy for the regeneration of supports. J Phys Chem C 115:6350–6359
Godage OS, Bucharskaya AB, Navolokin NA, Maslyakova GN, German SV, Gorin DA (2017) The magnetite nanoparticles in theranostic applications. J Nanomed Res 5(3):00119
Revia RA, Zhang M (2016) Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today 19:157–168
Borges J, Campiña JM, Silva AF (2013) Chitosan biopolymer-F(ab´)2 immunoconjugate films for enhanced antigen recognition. J Mater Chem B 1:500–511
Krishna R, Dias C, Ventura J, Titus E (2016) Green and facile decoration of Fe3O4 nanoparticles on reduced graphene oxide. Mater Today Proc 3:2807–2813
Krishna R, Titus E, Costa LC, Menezes JCJMDS, Correia MRP, Pinto S, Ventura J, Araújo JP, Cavaleiro JAS, Gracio JJA (2012) Facile synthesis of hydrogenated reduced graphene oxide via hydrogen spillover mechanism. J Mater Chem 22:10457–10459
Wang J, Meng G, Tao K, Feng M, Zhao X, Li Z, Xu H, Xia D, Lu JR (2012) Immobilization of lipases on alkyl silane modified magnetic nanoparticles: effect of alkyl chain length on enzyme activity. PLoS One 7:e43478
Li BJ, Cao HQ (2011) ZnO@graphene composite with enhanced performance for the removal of dye from water. J Mater Chem 21:3346–3349
Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG (1991) The handbook of infrared and Raman characteristic frequencies of organic molecules, 1st edn. Academic Press, London
Unnikrishnan B, Palanisamy S, Chen SM (2013) A simple electrochemical approach to fabricate a glucose biosensor based on graphene-glucose oxidase biocomposite. Biosens Bioelectron 39:70–75
Narayanan TN, Liu Z, Lakshmy PR, Gao W, Nagaoka Y, Kumar DS, Lou J, Vajtai R, Ajayan PM (2012) Synthesis of reduced graphene oxide-Fe3O4 multifunctional freestanding membranes and their temperature dependent electronic transport properties. Carbon 50:1338–1345
Acik M, Lee G, Mattevi C, Chhowalla M, Cho K, Chabal YJ (2010) Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat Mater 9:840–845
Bharath G, Madhu R, Chen SM, Veermani V, Balamurugan A, Mangalaraj D, Ponpandian N (2015) Enzymatic electrochemical glucose biosensensor by mesoporous 1D hydroxyapatite-on-2D reduced graphene oxide. J Mater Chem B 3:1360–1370
Ghasemzadeh MA, Abdollahi-Basir MH, Babaei M (2015) Fe3O4@SiO2-NH2 core-shell nanocomposite as an efficient and green catalyst for the multicomponent synthesis of highly substituted chromeno[2,3-b] pyridines in aqueous ethanol media. Green Chem Lett Rev 8:40–49
Bagbi Y, Sarswat A, Mohan D, Pandey A, Solanki PR (2016) Lead (Pb2+) adsorption by monodispersed magnetite nanoparticles: surface analysis and effects of solution chemistry. J Environ Chem Eng 4:4237–4247
Li SS, Tu KH, Lin CC, Chen CW, Chhowalla M (2010) Solution-processable graphene oxide as an efficient hole transport layer in polymer solar cells. ACS Nano 4:3169–3174
Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Spencer Williams E, Brooks BW (2015) Global assessment of bisphenol A in the environment: review and analysis of its occurrence and bioaccumulation. Dose Response July–September: 1–29
Biedermann-Brem S, Grob K (2009) Release of bisphenol A from polycarbonate baby bottles: water hardness as the most relevant factor. Eur Food Res Technol 228(5):679–684
Biles JE, McNeal TP, Begley TH, Hollifield HC (1997) Determination of bisphenol-A in reusable polycarbonate food-contact plastics and migration to food-simulating liquids. J Agric Food Chem 45:3541–3544
Pan D, Gu Y, Lan H, Sun Y, Gao H (2015) Functional graphene-gold nano-composite fabricated electrochemical biosensor for direct and rapid detection of bisphenol A. Anal Chim Acta 853:297–302
Wu L, Deng D, Jin J, Lu X, Chen J (2012) Nanographene-based tyrosinase biosensor for rapid detection of bisphenol A. Biosens Bioelectron 35:193–199
Alkasir RS, Ganesana M, Won YH, Stanciu L, Andreescu S (2010) Enzyme functionalized nanoparticles for electrochemical biosensors: a comparative study with applications for the detection of bisphenol A. Biosens Bioelectron 26:43–49
Acknowledgments
We warmly thank Pr. Elby Titus and Dr. Rahul Krishna for their kind donation of a sample of the hybrid conjugate.
Funding
This work was supported by European (FEDER) and National Portuguese funds (COMPETE and POPH programs; Pest-C/EQB/LA0006/2013, PTDC/EME-MFE/103051). PMVF and JMC acknowledge the Fundacão para a Ciência e a Tecnologia de Portugal (FCT) for the concession of a doctoral and a post-doctoral grant (SFRH/BD/111274/2015 and SFRH/BPD/109537/2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2626 kb)
Rights and permissions
About this article
Cite this article
Fernandes, P.M.V., Campiña, J.M. & Silva, A.F. A layered nanocomposite of laccase, chitosan, and Fe3O4 nanoparticles-reduced graphene oxide for the nanomolar electrochemical detection of bisphenol A. Microchim Acta 187, 262 (2020). https://doi.org/10.1007/s00604-020-4223-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4223-x