Abstract
A carbon nanotube (CNT)–mediated antibody-free suspension array (CASA) by integration of functionalized CNTs and aptamer (Apt) into xMAP® technology for simultaneous determination of typical endocrine-disrupting chemicals (EDCs) was developed . The interaction between CNTs and Apt acts as an effective and straightforward signal recognition, transformation, and amplification strategy. The amino-functionalized CNTs are covalently modified on the carboxyl-functionalized magnetic bead (MB) and further physically bridging with biotinylated Apt. CNTs on the surface of MBs not only increase the amount of Apt for target binding and signal amplification but also maintain the biological activity of Apt. After magnetic separation, the encoded MB address was distinguished and the concentration of the target in the liquid was negatively correlated with median fluorescence intensity. A series of environmental water samples were analysed by CASA, traditional immuno-SA, and competitive inhibition enzyme-linked immunosorbent assay for validation. The results obtained using CASA well matched for the multiplexed detection of various targets with dynamic concentration range from 6.40 × 10−5 to 4.00 μg L−1 within 1 h. The method also confirmed good selectively, accuracy, and consistency with high-performance liquid chromatography.

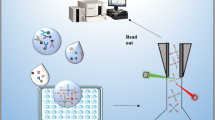
Schematic representation of amino-functionalized carbon nanotube (CNT)–bridged antibody-free suspension array for detecting of three typical endocrine-disrupting chemicals. The amino-functionalized CNTs are covalently modified and further physically interacted with biotinylated aptamer featuring in the recognition and binding with the target of interest.






Similar content being viewed by others
References
Liu H, Yang X, Lu R (2016) Development of classification model and QSAR model for predicting binding affinity of endocrine disrupting chemicals to human sex hormone-binding globulin. Chemosphere 156:1–7
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC (2009) Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev 30(4):293–342
Rachon D (2015) Endocrine disrupting chemicals (EDCs) and female cancer: informing the patients. Rev Endocr Metab Disord 16(4):359–364
Jacobson JL, Jacobson SW (1996) Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med 335(11):783–789
Liu S, Zhang S, Qu C, Liu W, Du Y (2010) The associations between the environmental exposure to polychlorinated biphenyls (PCBs) and breast cancer risk and progression. Sci China Chem 53(5):974–979
Hauser R, Williams P, Altshul L, Calafat AM (2005) Evidence of interaction between polychlorinatid biphenyls and phthalates in relation to human sperm motility. Environ Health Perspect 113(4):425–430
McAuliffe ME, Williams PL, Korrick SA, Altshul LM, Perry MJ (2012) Environmental exposure to polychlorinated biphenyls and p,p′-DDE and sperm sex-chromosome disomy. Environ Health Perspect 120(4):535–540
Liu N, Gao Z, Ma H, Su P, Ma X, Li X, Ou G (2013) Simultaneous and rapid detection of multiple pesticide and veterinary drug residues by suspension array technology. Biosens Bioelectron 41(Supplement C):710–716
Liu N, Liang W, Ma X, Li X, Ning B, Cheng C, Ou G, Wang B, Zhang J, Gao Z (2013) Simultaneous and combined detection of multiple tumor biomarkers for prostate cancer in human serum by suspension array technology. Biosens Bioelectron 47:92–98
Sun Z, Peng Y, Zhang M, Wang K, Bai J, Li X, Ning B, Gao Z (2014) Simultaneous and highly sensitive detection of six different foodborne pathogens by high-throughput suspension array technology. Food Control 40(2):300–309
Liu N, Zheng F, Zheng X (2017) Detection of biomarkers of acute myocardial infarction by high-throughput suspension array technology in serum sample. Bioanalysis 10(1):47–58
Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, DiMattia MA, Zaal KJM, Sanchez GAM, Kim H, Chapelle D, Plass N, Huang Y, Villarino AV, Biancotto A, Fleisher TA, Duncan JA, O’Shea JJ, Benseler S, Grom A, Deng Z, Laxer RM, Goldbach-Mansky R (2014) An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 46:1140–1146
Song X, Wang D, Kim M (2019) Immunoliposome-based fluorometric patulin assay by using immunomagnetic nanoparticles. Microchim Acta 186(12):834
Tian R, Luo M, Li J (2018) Spontaneous protein desorption from self-assembled monolayer (SAM)-coated gold nanoparticles. Phys Chem Chem Phys 20(1):68–74
Tan W, Donovan MJ, Jiang J (2013) Aptamers from cell-based selection for bioanalytical applications. Chem Rev 113(4):2842–2862
Chen Y, Zhou S, Li L, Zhu J (2017) Nanomaterials-based sensitive electrochemiluminescence biosensing. Nano Today 12:98–115
Jiang X, Wang H, Wang H, Zhuo Y, Yuan R, Chai Y (2017) Electrochemiluminescence biosensor based on 3-D DNA nanomachine signal probe powered by protein-aptamer binding complex for ultrasensitive mucin 1 detection. Anal Chem 89(7):4280–4286
Nguyen V-T, Kwon YS, Gu MB (2017) Aptamer-based environmental biosensors for small molecule contaminants. Curr Opin Biotechnol 45:15–23
Sze JYY, Ivanov AP, Cass AEG, Edel JB (2017) Single molecule multiplexed nanopore protein screening in human serum using aptamer modified DNA carriers. Nat Commun 8:1552
Jiang Y, Shi M, Liu Y, Wan S, Cui C, Zhang L, Tan W (2017) Aptamer/AuNP biosensor for colorimetric profiling of exosomal proteins. Angew Chem Int Ed 56(39):11916–11920
Hu X, Wang Y, Tan Y, Wang J, Liu H, Wang Y, Yang S, Shi M, Zhao S, Zhang Y, Yuan Q (2017) A difunctional regeneration scaffold for knee repair based on aptamer-directed cell recruitment. Adv Mater 29(15):1605235
Liu Q, Jin C, Wang Y, Fang X, Zhang X, Chen Z, Tan W (2014) Aptamer-conjugated nanomaterials for specific cancer cell recognition and targeted cancer therapy. Npg Asia Mater 6:e95
Xu Y, Wang H, Luan C, Liu Y, Chen B, Zhao Y (2018) Aptamer-based hydrogel barcodes for the capture and detection of multiple types of pathogenic bacteria. Biosens Bioelectron 100:404–410
Lu C, Huang Z, Liu B, Liu Y, Ying Y, Liu J (2017) Poly-cytosine DNA as a high-affinity ligand for inorganic nanomaterials. Angew Chem Int Ed 56(22):6208–6212
Panes-Ruiz LA, Shaygan M, Fu Y, Liu Y, Khavrus V, Oswald S, Gemming T, Baraban L, Bezugly V, Cuniberti G (2018) Toward highly sensitive and energy efficient ammonia gas detection with modified single-walled carbon nanotubes at room temperature. ACS Sens 3(1):79–86
Azzouz A, Kailasa SK, Kumar P, Ballesteros E, Kim K-H (2019) Advances in functional nanomaterial-based electrochemical techniques for screening of endocrine disrupting chemicals in various sample matrices. TrAC-Trend Anal Chem 113:256–279
Zhang X, Yu Z, Wang C, Zarrouk D, Seo J-W T, Cheng JC, Buchan AD, Takei K, Zhao Y, Ager JW, Zhang J, Hettick M, Hersam MC, Pisano AP, Fearing RS, Javey A (2014) Photoactuators and motors based on carbon nanotubes with selective chirality distributions. Nat Commun 5:2983
Lei T, Chen X, Pitner G, Wong HSP, Bao Z (2016) Removable and recyclable conjugated polymers for highly selective and high-yield dispersion and release of low-cost carbon nanotubes. J Am Chem Soc 138(3):802–805
Zheng M, Jagota A, Strano MS, Santos AP, Barone P, Chou SG, Diner BA, Dresselhaus MS, McLean RS, Onoa GB, Samsonidze GG, Semke ED, Usrey M, Walls DJ (2003) Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science 302(5650):1545–1548
Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG (2003) DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater 2(5):338–342
Tiwari JN, Vij V, Kemp KC, Kim KS (2016) Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 10(1):46–80
Liu Y, Liu N, Ma X, Li X, Ma J, Li Y, Zhou Z, Gao Z (2015) Highly specific detection of thrombin using an aptamer-based suspension array and the interaction analysis via microscale thermophoresis. Analyst 140(8):2762–2770
Niazi JH, Verma SK, Niazi S, Qureshi A (2015) In vitro HER2 protein-induced affinity dissociation of carbon nanotube-wrapped anti-HER2 aptamers for HER2 protein detection. Analyst 140(1):243–249
Yang R, Tang Z, Yan J, Kang H, Kim Y, Zhu Z, Tan W (2008) Noncovalent assembly of carbon nanotubes and single-stranded DNA: an effective sensing platform for probing biomolecular interactions. Anal Chem 80(19):7408–7413
Zhao Y, Zhao X, Tang B, Xu W, Li J, Hu J, Gu Z (2010) Quantum-dot-tagged bioresponsive hydrogel suspension array for multiplex label-free DNA detection. Adv Funct Mater 20(6):976–982
Wang H, Xu Q, Shang L, Wang J, Rong F, Gu Z, Zhao Y (2016) Boronate affinity molecularly imprinted inverse opal particles for multiple label-free bioassays. Chem Commun 52(16):3296–3299
Zheng F, Cheng Y, Wang J, Lu J, Zhang B, Zhao Y, Gu Z (2014) Aptamer-functionalized barcode particles for the capture and detection of multiple types of circulating tumor cells. Adv Mater 26(43):7333–7338
Zhu Y, Deng P, Zhong D (2015) Derivatization methods for LC–MS analysis of endogenous compounds. Bioanalysis 7(19):2557–2581
Barber LB, Vajda AM, Douville C, Norris DO, Writer JH (2012) Fish endocrine disruption responses to a major wastewater treatment facility upgrade. Environ Sci Technol 46(4):2121–2131
Xu Y, Wang H, Luan C, Fu F, Chen B, Liu H, Zhao Y (2018) Porous hydrogel encapsulated photonic barcodes for multiplex microRNA quantification. Adv Funct Mater 28(1):1704458
Funding
The authors gratefully acknowledge the financial support by the National Natural Science Foundation of China (No. 81872584, 81273078, 81472941, 81671784, and 21505027), the National 863 Young Scientist Program (No. 2015AA020940), the Beijing Nova Program (Z181100006218017), the Natural Science Foundation of Tianjin City (No. 16JCZDJC39500), the Science and Technology Major Project of Tianjin City (No. 16ZXHLSY00130), the Natural Science Foundation of Guangdong Province (2016A030313138), and the Key Projects of Guangzhou Science and Technology Program (No. 201704020056).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, N., Wu, J., Xianyu, Y. et al. Carbon nanotube–mediated antibody-free suspension array for determination of typical endocrine-disrupting chemicals. Microchim Acta 187, 202 (2020). https://doi.org/10.1007/s00604-020-4181-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4181-3