Abstract
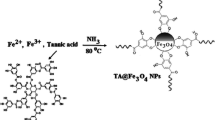
Carbonaceous shell-coated γ-Fe2O3 nanoparticles (γ-Fe2O3@CNM) were synthesized from glucose caramelization and used as a novel magnetic solid-phase extraction medium for malachite green and crystal violet in environmental water. Malachite green and crystal violet were absorbed on to γ-Fe2O3@CNM by electrostatic and π-interactions. The morphologies, pore structures, surface functional groups, and magnetic properties of γ-Fe2O3@CNM were characterized by TEM, FTIR, hysteresis regression, Brunauer-Emmet-Teller analysis, zeta potential, XPS, and XRD. The magnetic solid-phase extraction procedure was optimized by extraction pH, absorption time, desorption solvent, and desorption time. The absorption capacities (qmax values) for malachite green and crystal violet were 34.2 and 27.9 mg g−1, respectively. After magnetic solid-phase extraction, malachite green and crystal violet were determined by LC-MS/MS. The analytical method was validated with a linear range of 0.02–20 ng mL−1, enrichment factor of 25.8 and 25.4, method detection limit of 0.004 ng mL−1, and intra-day precisions of 2.1% and 2.6% for malachite green and crystal violet, respectively. The relative recovery was found to be 73.4–101.5% for malachite green and 83.1–102.7% for crystal violet upon the application of the magnetic solid-phase extraction method to real water samples from lake, spring, sea, fishpond, and industrial waste.

Caramelized-carbon-coated magnetic nanoparticles are used as novel extraction medium based on electrostatic and π-interactions. It is porous, amphiphilic, electronegative, magnetically strong, and features abundant absorption site. These characteristics stimulate mass transfer and result in a useful MSPE method in environmental analysis.



Similar content being viewed by others
References
Chen L, Wang T, Tong J (2011) Application of derivatized magnetic materials to the separation and the preconcentration of pollutants in water samples. Trac-Trend Anal Chem 30:1095–1108. https://doi.org/10.1016/j.trac.2011.02.013
Aguilar-Arteaga K, Rodriguez JA, Barrado E (2010) Magnetic solids in analytical chemistry: a review. Anal Chim Acta 674:157–165. https://doi.org/10.1016/j.aca.2010.06.043
Wierucka M, Biziuk M (2014) Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. Trac-Trend Anal Chem 59:50–58. https://doi.org/10.1016/j.trac.2014.04.007
Safari M, Yamini Y, Tahmasebi E, Ebrahimpour B (2016) Magnetic nanoparticle assisted supramolecular solvent extraction of triazine herbicides prior to their determination by HPLC with UV detection. Microchim Acta 183:203–210. https://doi.org/10.1007/s00604-015-1607-4
Gao L, Chen L, Li X (2015) Magnetic molecularly imprinted polymers based on carbon nanotubes for extraction of carbamates. Microchim Acta 182:781–787. https://doi.org/10.1007/s00604-014-1388-1
Wang Q, Wang Y, Zhang Z, Tong Y, Zhang L (2017) Waxberry-like magnetic porous carbon composites prepared from a nickel-organic framework for solid-phase extraction of fluoroquinolones. Microchim Acta 184:4107–4115. https://doi.org/10.1007/s00604-017-2438-2
Azzouz A, Kailasa SK, Lee SS, Rascon AJ, Ballesteros E, Zhang M, Kim K-H (2018) Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. Trac-Trend Anal Chem 108:347–369. https://doi.org/10.1016/j.trac.2018.08.009
Hemmati M, Rajabi M, Asghari A (2018) Magnetic nanoparticle based solid-phase extraction of heavy metal ions: a review on recent advances. Microchim Acta 185:160. https://doi.org/10.1007/s00604-018-2670-4
Mashkani M, Mehdinia A, Jabbari A, Bide Y, Nabid MR (2018) Preconcentration and extraction of lead ions in vegetable and water samples by N-doped carbon quantum dot conjugated with Fe3O4 as a green and facial adsorbent. Food Chem 239:1019–1026. https://doi.org/10.1016/j.foodchem.2017.07.042
Hu C, Yang Z, Yan F, Sun B (2019) Extraction of the toluene exposure biomarkers hippuric acid and methylhippuric acid using a magnetic molecularly imprinted polymer, and their quantitation by LC-MS/MS. Microchim Acta 186:135. https://doi.org/10.1007/s00604-019-3239-6
Chiang C, Chen W, Chang H (2011) Nanoparticle-based mass spectrometry for the analysis of biomolecules. Chem Soc Rev 40:1269–1281. https://doi.org/10.1039/C0CS00050G
Su J, He X, Chen L, Zhang Y (2018) Adenosine phosphate functionalized magnetic mesoporous graphene oxide nanocomposite for highly selective enrichment of phosphopeptides. ACS Sustain Chem Eng 6:2188–2196. https://doi.org/10.1021/acssuschemeng.7b03607
Villanueva J, Philip J, Entenberg D, Chaparro CA, Tanwar MK, Holland EC, Tempst P (2004) Serum peptide profiling by magnetic particle-assisted, automated sample processing and MALDI-TOF mass spectrometry. Anal Chem 76:1560–1570. https://doi.org/10.1021/ac0352171
Khajeh M, Laurent S, Dastafkan K (2013) Nanoadsorbents: classification, preparation, and applications (with emphasis on aqueous media). Chem Rev 113:7728–7768. https://doi.org/10.1021/cr400086v
Li D, Zhu J, Wang M, Bi W, Huang X, Chen DDY (2017) Extraction of trace polychlorinated biphenyls in environmental waters by well-dispersed velvet-like magnetic carbon nitride nanocomposites. J Chromatogr A 1491:27–35. https://doi.org/10.1016/j.chroma.2017.02.048
Tang S, Sun J, Xia D, Zang B, Gao Y, Chen C, Shen W, Lee HK (2019) In-syringe extraction using compressible and self-recoverable, amphiphilic graphene aerogel as sorbent for determination of phenols. Talanta 195:165–172. https://doi.org/10.1016/j.talanta.2018.11.038
Mousavi M, Habibi-Yangjeh A (2016) Magnetically separable ternary g-C3N4/Fe3O4/BiOI nanocomposites: novel visible-light-driven photocatalysts based on graphitic carbon nitride. J Colloid Interface Sci 465:83–92. https://doi.org/10.1016/j.jcis.2015.11.057
Guo W, Qi Y, Zhang Y, Ma L, Yu D, Zhan J (2016) Biocompatible caramelized carbonaceous nanospheres supported paramagnetic ultrathin manganese oxide nanosheets via self-sacrificing reduction as a MRI contrast agent for liver imaging. Carbon 110:321–329. https://doi.org/10.1016/j.carbon.2016.09.030
Kaneti YV, Chen C, Liu M, Wang X, Yang J, Taylor RA, Jiang X, Yu A (2015) Carbon-coated gold nanorods: a facile route to biocompatible materials for photothermal applications. ACS Appl Mater Interfaces 7:25658–25668. https://doi.org/10.1021/acsami.5b07975
Xia H, Wan Y, Yuan G, Fu Y, Wang X (2013) Fe3O4/carbon core-shell nanotubes as promising anode materials for lithium-ion batteries. J Power Sources 241:486–493. https://doi.org/10.1016/j.jpowsour.2013.04.126
Xiang Y, Li N, Guo L, Wang H, Sun H, Li R, Ma L, Qi Y, Zhan J, Yu D (2018) Biocompatible and pH-sensitive MnO-loaded carbonaceous nanospheres (MnO@CNSs): a theranostic agent for magnetic resonance imaging-guided photothermal therapy. Carbon 136:113–124. https://doi.org/10.1016/j.carbon.2018.04.058
Vinodha G, Shima PD, Cindrella L (2019) Mesoporous magnetite nanoparticle-decorated graphene oxide nanosheets for efficient electrochemical detection of hydrazine. J Mater Sci 54:4073–4088. https://doi.org/10.1007/s10853-018-3145-z
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254:2441–2449. https://doi.org/10.1016/j.apsusc.2007.09.063
Fujii T, de Groot FMF, Sawatzky GA, Voogt FC, Hibma T, Okada K (1999) In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys Rev B 59:3195–3202. https://doi.org/10.1103/PhysRevB.59.3195
Li Z, Li X, Zong Y, Tan G, Sun Y, Lan Y, He M, Ren Z, Zheng X (2017) Solvothermal synthesis of nitrogen-doped graphene decorated by superparamagnetic Fe3O4 nanoparticles and their applications as enhanced synergistic microwave absorbers. Carbon 115:493–502. https://doi.org/10.1016/j.carbon.2017.01.036
Wilson D, Langell MA (2014) XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl Surf Sci 303:6–13. https://doi.org/10.1016/j.apsusc.2014.02.006
Wang W, Tang B, Ju B, Gao Z, Xiu J, Zhang S (2017) Fe3O4-functionalized graphene nanosheet embedded phase change material composites: efficient magnetic- and sunlight-driven energy conversion and storage. J Mater Chem A 5:958–968. https://doi.org/10.1039/C6TA07144A
Zhou Z, Fu Y, Qin Q, Lu X, Shi X, Zhao C, Xu G (2018) Synthesis of magnetic mesoporous metal-organic framework-5 for the effective enrichment of malachite green and crystal violet in fish samples. J Chromatogr A 1560:19–25. https://doi.org/10.1016/j.chroma.2018.05.016
Wei X, Wang Y, Chen J, Xu P, Xu W, Ni R, Meng J, Zhou Y (2019) Poly (deep eutectic solvent)-functionalized magnetic metal-organic framework composites coupled with solid-phase extraction for the selective separation of cationic dyes. Anal Chimi Acta 1056:47–61. https://doi.org/10.1016/j.aca.2018.12.049
Zhang L, Zhang Y, Tang Y, Li X, Zhang X, Li C, Xu S (2018) Magnetic solid-phase extraction based on Fe3O4/graphene oxide nanoparticles for the determination of malachite green and crystal violet in environmental water samples by HPLC. Int J Environ Anal Chem 98:215–228. https://doi.org/10.1080/03067319.2018.1441837
Liang N, Hou X, Huang P, Jiang C, Chen L, Zhao L (2017) Ionic liquid-based dispersive liquid-liquid microextraction combined with functionalized magnetic nanoparticle solid-phase extraction for determination of industrial dyes in water. Sci Rep 7:13844. https://doi.org/10.1038/s41598-017-14098-1
Zhao J, Wei D, Yang Y (2016) Magnetic solid-phase extraction for determination of the total malachite green, gentian violet and leucomalachite green, leucogentian violet in aquaculture water by high-performance liquid chromatography with fluorescence detection. J Sep Sci 39:2347–2355. https://doi.org/10.1002/jssc.201501363
Wang Y, Chen L (2015) Analysis of malachite green in aquatic products by carbon nanotube-based molecularly imprinted-matrix solid phase dispersion. J Chromatogr B 1002:98–106. https://doi.org/10.1016/j.jchromb.2015.08.002
Tan L, Chen K, He R, Peng R, Huang C (2016) Temperature sensitive molecularly imprinted microspheres for solid-phase dispersion extraction of malachite green, crystal violet and their leuko metabolites. Microchim Acta 183:2991–2999. https://doi.org/10.1007/s00604-016-1947-8
Li L, Lin Z, Chen X, Zhang H, Lin Y, Lai Z, Huang Z (2015) Molecularly imprinted polymers for extraction of malachite green from fish samples prior to its determination by HPLC. Microchim Acta 182:1791–1796. https://doi.org/10.1007/s00604-015-1513-9
Funding
This work is based upon the work supported by the National Natural Science Foundation of China (NSFC) (21705093, 21603119, 21876099, 21750110438, and 21575077), the Taishan Scholars Project of Shandong Province (ts201712011), the Natural Science Foundation of Shandong Province (ZR2016BQ09 and ZR2017BB061), the Natural Science Foundation of Jiangsu Province (BK20170396), the Science and Technology Development Plans of Shandong Province (ZR2017ZC0227), the Young Scholars Program of Shandong University (YSPSDU, 2018WLJH48) and the Fundamental Research Funds of Shandong University (2017 TB003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 458 kb)
Rights and permissions
About this article
Cite this article
Li, N., Li, R., Song, Y. et al. Caramelized carbonaceous shell-coated γ-Fe2O3 as a magnetic solid-phase extraction sorbent for LC-MS/MS analysis of triphenylmethane dyes. Microchim Acta 187, 371 (2020). https://doi.org/10.1007/s00604-020-04346-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04346-z