Abstract
Nitrogen-doped carbon dots (NCDs) were synthesized via hydrothermal treatment of vitamin B1 and triethylamine. The NCDs exhibit strong blue fluorescence (with a peak at 437 nm at an excitation wavelength of 370 nm), good water solubility and excellent fluorescence stability in the pH 3~12 range, at ionic strengths between 0.01 and 1 M, and under UV illumination for 6 h, as well as incubation temperature of 15~60 °C. The nanoparticles respond selectively and sensitively to trace concentrations of perfluorooctane sulfonate (PFOS) through electrostatic interactions between PFOS and NCDs. This is accompanied by the aggregation of NCDs to yield enhanced fluorescence. The nanoprobe has high selectivity for PFOS even in presence of other common ions such as metal ions, anions, and structural analogues such as surfactants. Under the optimal conditions, the response is linear in the 0.3 to 160 nM PFOS concentration range with a detection limit of 0.3 nM. Satisfactory results were achieved for determination of PFOS in spiked real water samples.

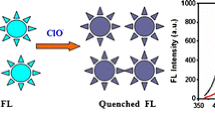
Schematic presentation of the synthetic route to nitrogen-doped carbon dots (NCDs) starting from vitamin B1 and triethylamine, and its application for selective and sensitive fluorometric determination of perfluorooctane sulfonate (PFOS).






Similar content being viewed by others
References
Giesy JP, Kannan K (2002) Perfluorochemical surfactants in the environment. Environ Sci Technol 36:146A–152A
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem Rev 114:4564–4601
Viberg H, Eriksson P (2011) Perfluorooctane Sulfonate and Perfluorooctanoic Acid[M], Reproductive and Developmental Toxicology, Elsevier
Sanganyado E, Rajput IR, Liu W (2018) Bioaccumulation of organic pollutants in indo-Pacific humpback dolphin: a review on current knowledge and future prospects. Environ Pollut 237:111–125
Yin N, Yang R, Liang S, Liang S, Hu B, Ruan T, Faiola F (2018) Evaluation of the early developmental neural toxicity of F-53B, as compared to PFOS, with an in vitro mouse stem cell differentiation model. Chemosphere 204:109–118
Armitage JM, Schenker U, Scheringer M, Martin JW, MacLeod M, Cousins IT (2009) Modeling the global fate and transport of perfluorooctane sulfonate (PFOS) and precursor compounds in relation to temporal trends in wildlife exposure. Environ Sci Technol 43:9274–9280
Daughton CG (2005) "Emerging" chemicals as pollutants in the environment: a 21st century perspective. Renew Resour J 23:6–23
Thanh W, Yawei W, Chunyang L, Yaqi C, Guibin J (2009) Perspectives on the inclusion of perfluorooctane sulfonate into the Stockholm convention on persistent organic pollutants. Environ Sci Technol 43:5171–5175
Environmental Protection Agency (EPA), Provisional Health Advisories for Perfluorooctanoric acid (PFOA) and Perfluorooctane Sulfonate (PFOS). http://water.epa.gov/action/advisories/drinking/upload/2009_01_15_criteria_drinking_pha_PFOA_PFOS.PDF. (Accessed July 2014)
European Commission, Directive 2013/39/EU of the European Paliament and of the council of 12 august 2013 amending directives 2000/60/EC and 2008/105/EC as regards priority Subatances in the field of water policy. Official Journal of the European Union, 2013, L226/1
Sun RWM, Tang L, Li J, Qian Z, Han T, Xu G (2018) Perfluorinated compounds in surface waters of Shanghai, China: source analysis and risk assessment. Ecotox Environ Safe 149:88–95
Zareitalabad P, Siemens J, Hamer M, Amelung W (2013) Amelung, Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater-a review on concentrations and distribution coefficients. Chemosphere 91:725–732
Zacs D, Bartkevics V (2016) Trace determination of perfluorooctane sulfonate and perfluorooctanoic acid in environmental samples (surface water, wastewater, biota, sediments, and sewage sludge) using liquid chromatography-orbitrap mass spectrometry. J Chromatogr A 1473:109–121
Munoz G, Labadie P, Geneste E, Pardon P, Tartu S, Chastel O, Budzinski H (2017) Biomonitoring of fluoroalkylated substances in Antarctica seabird plasma: development and validation of a fast and rugged method using on-line concentration liquid chromatography tandem mass spectrometry. J Chromatogr A 1513:107–117
Lin M, Yang Y, Liu Y, Sun MX (2013) Trace analysis of perfluorooctane sulfonic acid in textile fabrics by gas chromatography/mass spectrometry with on-column derivatization. Chinese J Anal Chem 41:888–892
Sun YP, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie SY (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128:7756–7757
Li H, Kang Z, Liu Y, Lee S-T (2012) Carbon nanodots: synthesis, properties and applications. J Mater Chem 22:24230–24253
Bandi R, Devulapalli NP, Dadigala R, Gangapuram BR, Guttena V (2018) Facile conversion of toxic cigarette butts to N,S-Codoped carbon dots and their application in fluorescent film, security ink, bioimaging, sensing and logic gate operation. ACS Omega 3:13454–13466
Ding H, Du F, Liu P, Chen Z, Shen J (2015) DNA–carbon dots function as fluorescent vehicles for drug delivery. ACS Appl Mater Inter 7:6889–6897
Wang L, Li M, Li W, Han Y, Liu Y, Li Z, Zhang B, Pan D (2018) Rationally designed efficient dual-mode colorimetric/fluorescence sensor based on carbon dots for detection of pH and Cu2+ ions. ACS Sustain Chem Eng 6:12668–12674
Moon BJ, Oh Y, Shin DH, Kim SJ, Lee SH, Kim TW, Park M, Bae S (2016) Facile and purification-free synthesis of nitrogenated amphiphilic graphitic carbon dots. Chem Mater 28:1481–1488
Yang S, Li Y, Wang S, Wang M, Chu M, Xia B (2018) Advances in the use of carbonaceous materials for the electrochemical determination of persistent organic pollutants. A review. Microchim Acta 185:112–125
Xiang GQ, Ren Y, Xia Y, Mao W, Fan C, Guo SY, Wang PP, Yang DH, He L, Jiang X (2017) Carbon-dot-based dual-emission silica nanoparticles as a ratiometric fluorescent probe for bisphenol a. Spectrochim Acta A 177:153–157
Liu G, Chen Z, Jiang X, Feng DQ, Zhao J, Fan D, Wang W (2016) In-situ hydrothermal synthesis of molecularly imprinted polymers coated carbon dots for fluorescent detection of bisphenol a. Sensor Actuat B-Chem 228:302–307
Wang Y, Ni P, Jiang S, Lu W, Li Z, Liu H, Lin J, Sun Y, Li Z (2018) Highly sensitive fluorometric determination of oxytetracycline based on carbon dots and Fe3O4 MNPs. Sensor Actuat B-Chem 254:1118–1124
Cheng Z, Dong H, Liang J, Zhang F, Chen X, Du L, Tan K (2019) Highly selective fluorescent visual detection of perfluorooctane sulfonate via blue fluorescent carbon dots and berberine chloride hydrate. Spectrochim Acta A 207:262–269
Chen Q, Zhu P, Xiong J, Gao L, Tan K (2019) A sensitive and selective triple-channel optical assay based on red-emissive carbon dots for the determination of PFOS. Microchem J 145:388–396
Jiao Z, Li J, Mo L, Liang J, Fan H (2018) A molecularly imprinted chitosan doped with carbon quantum dots for fluorometric determination of perfluorooctane sulfonate. Microchim Acta 185:473–481
Shi L, Yang JH, Zeng HB, Chen YM, Yang SC, Wu C, Zeng H, Yoshihito O, Zhang Q (2016) Carbon dots with high fluorescence quantum yield: the fluorescence originates from organic fluorophores. Nanoscale 8:14374–14378
Jana J, Ganguly M, Chandrakumar KR, Mohan RG, Pal T (2016) Boron precursor dependent evolution of differently emitting carbon dots. Langmuir 33:573–584
Wu M, Wang Y, Wu W, Hu C, Wang X, Zheng J, Li Z, Jiang B, Qiu J (2014) Preparation of functionalized water-soluble photoluminescent carbon quantum dots from petroleum coke. Carbon 78:480–489
Reckmeier CJ, Wang Y, Zboril R, Rogach AL (2016) Influence of doping and temperature on solvatochromic shifts in optical spectra of carbon dots. J Phys Chem C 120:10591–10604
Haijuan Z, Yonglei C, Meijuan L, Laifang X, Shengda Q, Hongli C, Xingguo C (2014) Solid-phase synthesis of highly fluorescent nitrogen-doped carbon dots for sensitive and selective probing ferric ions in living cells. Anal Chem 86:9846–9852
Wu F, Zhu J, Tan K (2012) Resonance light scattering spectra of perfluorooctane sulfonate-protein system and its analytical application. Chinese J Appl Chem 29:969–973
Zuo P, Lu X, Sun Z, Guo Y, Hua H (2016) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183:519–542
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21665018 and 21366024), Natural Science Foundation of Jiangxi Province (No. 20181BAB203014) and Foundation of Jiangxi Educational Committee (No. GJJ160724). This work was also supported by the “Graduate Student Innovation Funds for the Nanchang Hangkong University (No.YC2018-S361)”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1) Nitrogen-doped carbon dots are a viable nanoprobe for PFOS detection
2) The detection strategy is based on fluorescence enhancement with LOD of 0.3 nM
3) The nanoprobe is sensitive and selectivity for PFOS detection in water samples
Electronic supplementary material
ESM 1
(DOC 2.02 mb)
Rights and permissions
About this article
Cite this article
Lin, L., Zhou, S., Guo, H. et al. Nitrogen-doped carbon dots as an effective fluorescence enhancing system for the determination of perfluorooctyl sulfonate. Microchim Acta 186, 380 (2019). https://doi.org/10.1007/s00604-019-3501-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3501-y