Abstract
The authors describe an aptamer based assay for the mycotoxin T-2. The method is making use of exponential isothermal amplification reaction (EXPAR) and fluorescent silver nanoclusters (AgNCs). Free T-2 and cDNA (which is a DNA that is partially complementary to the aptamer) compete for binding to aptamer-modified magnetic beads. The cDNA collected by magnetic separation can be used as a primer to trigger EXPAR to obtain ssDNA. The C-base-rich ssDNA binds and reduces Ag(I) ion to form fluorescent AgNCs. Fluorescence is measured at excitation/emission wavelengths of 480/525 nm. T-2 can be detected by fluorometry with a detection limit as low as 30 fg·mL−1. The method was applied to analyse spiked oat and corn, and the average recoveries ranged from 97.3 to 102.3% and from 95.9 to 107.5%, respectively. The results were in good agreement with data of the commercial ELISA kit. The assay is highly sensitive, has a wide analytical range, good specificity and reliable operation. It provides a promising alternative for the standard method for quantitative detection of T-2.

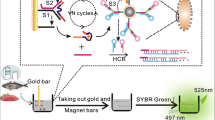
Schematic presentation of fluorometric assay for T-2 based on aptamer-functionalized magnetic beads exponential, isothermal amplification reaction (EXPAR) and fluorescent silver nanoclusters (AgNCs).




Similar content being viewed by others
References
Gao X, Cao W, Chen M, Xiong H, Zhang X, Wang S (2014) A high sensitivity electrochemical sensor based on Fe3+-ion molecularly imprinted film for the detection of T-2 toxin. In: Electroanalysis
Henri O. Arola (2017) A simple and specific noncompetitive ELISA method for HT-2 toxin detection. Toxins
Jiafa Hou (2016) Toxicity and oxidative stress induced by T-2 toxin and HT-2 toxin in broilers and broiler hepatocytes. Food Chem Toxicol
Li D, Han J, Guo X, Qu C, Yu F, Wu X (2016) The effects of T-2 toxin on the prevalence and development of Kashin–Beck disease in China: a meta-analysis and systematic review. Toxicol Research 5(3):731–751
McCormick SP, Kato T, Maragos CM, Busman M, Lattanzio VM, Galaverna G, Dall-Asta C, Crich D, Price NP, Kurtzman CP (2015) Anomericity of T-2 toxin-glucoside: masked mycotoxin in cereal crops. J Agric Food Chem 63(2):731–738
Visconti A (2005) Analysis of T-2 and HT-2 toxins in cereal grains by immunoaffinity clean-up and liquid chromatography with fluorescence detection. J Chromatogr A 1075:151–158
Omurtag GZ, YazıcıoǧLu D (2000) Determination of T-2 toxin in grain and grain products by HPLC and TLC. J Environ Sci Health B 35(6):797–807
Zou Z, He Z, Li H, Han P, Tang J, Xi C, Li Y, Zhang L, Li X (2012) Development and application of a method for the analysis of two trichothecenes: Deoxynivalenol and T-2 toxin in meat in China by HPLC–MS/MS. Meat Sci 90(3):613–617
Weijun K, Xiaofei Z, Honghong S, Zhen OY, Meihua Y (2012) Validation of a gas chromatography-electron capture detection of T-2 and HT-2 toxins in Chinese herbal medicines and related products after immunoaffinity column clean-up and pre-column derivatization. Food Chem 132(1):574–581
Meneely JP (2010) A rapid optical immunoassay for the screening of T-2 and HT-2 toxin in cereals and maize-based baby food. Talanta 81:630–636
Widiastuti R, Indraningsih (2013) Detection of deoxynivalenol and T-2 toxin in feeds by gas chromatography with Electron captured detector. IJAVS 14(3)
Sun X, Wang L, Zhao M, Zhao C, Liu S (2016) An autocatalytic DNA machine with autonomous target recycling and cascade circular exponential amplification for one-pot, isothermal and ultrasensitive nucleic acid detection. Chem Commun (Camb) 52(74):11108–11111
Chen L, Sha L, Qiu Y, Wang G, Jiang H, Zhang X (2015) An amplified electrochemical assay based on hybridization chain reactions and catalysis of silver nanoclusters. Nanoscale 7(7):3300–3308
Jia H, Li Z, Liu C, Cheng Y (2010) Ultrasensitive detection of microRNAs by exponential isothermal amplification. Angew Chem Int Ed 49(32):5498–5501
Liu H, Tian T, Zhang Y, Ding L, Yu J, Yan M (2017) Sensitive and rapid detection of microRNAs using hairpin probes-mediated exponential isothermal amplification. Biosens Bioelectron 89(Pt 2):710–714
Liu YQ, Zhang M, Yin BC, Ye BC (2012) Attomolar ultrasensitive microRNA detection by DNA-scaffolded silver-nanocluster probe based on isothermal amplification. Anal Chem 84(12):5165–5169
Reid MS, Le XC, Zhang H (2018) Exponential isothermal amplification of nucleic acids and amplified assays for proteins, cells, and enzyme activities. Angew Chem Int Ed 57:11856–11866
Van Ness J, Van Ness LK, Galas DJ (2003) Isothermal reactions for the amplification of oligonucleotides. Proc Natl Acad Sci U S A 100(8):4504–4509
Qu F, Liu Y, Kong R, You J (2017) A versatile DNA detection scheme based on the quenching of fluorescent silver nanoclusters by MoS2 nanosheets: application to aptamer-based determination of hepatitis B virus and of dopamine. Microchim Acta 184(11):4417–4424
Khan IM, Zhao S, Niazi S, Mohsin A, Shoaib M, Duan N, Wu S, Wang Z (2018) Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent detection of T-2 toxin. Sensors Actuators B Chem 277:328–335
Guo L, Chen D, Yang M (2017) DNA-templated silver nanoclusters for fluorometric determination of the activity and inhibition of alkaline phosphatase. Microchim Acta 184(7):2165–2170
Braun E, Eichen Y, Sivan U, Ben-Yoseph G (1998) DNA-templated assembly and electrode attachment of a conducting silver wire. Nature 391:775–778
Liu S, Pang S (2018) A dual-model strategy for fluorometric determination of ascorbic acid and of ascorbic acid oxidase activity by using DNA-templated gold-silver nanoclusters. Microchim Acta 185(9):426
Lee ST, Beaumont D, Su XD, Muthoosamy K, New SY (2018) Formulation of DNA chimera templates: effects on emission behavior of silver nanoclusters and sensing. Anal Chim Acta 1010:62–68
Lin X, Liu Y, Deng J, Lyu Y, Qian P, Li Y, Wang S (2018) Multiple advanced logic gates made of DNA-ag nanocluster and the application for intelligent detection of pathogenic bacterial genes. Chem Sci 9(7):1774–1781
Obliosca JM, Liu C, Yeh HC (2013) Fluorescent silver nanoclusters as DNA probes. Nanoscale 5(18):8443–8461
Pascale M, Panzarini G, Visconti A (2012) Determination of HT-2 and T-2 toxins in oats and wheat by ultra-performance liquid chromatography with photodiode array detection. Talanta 89(2):231–236
Zhang X, Wu C, Wen K, Jiang H, Shen J, Zhang S, Wang Z (2015) Comparison of fluorescent microspheres and colloidal gold as labels in lateral flow Immunochromatographic assays for the detection of T-2 toxin. Molecules 21(1):27–36
Petrakova AV, Urusova AE, Voznyak MV, Zherdeva AV, Dzantiev BB (2015) Immunochromatographic test system for the detection of T-2 toxin. Prikl Biokhim Mikrobiol 51(6):616–623
Sun Y, Zhang G, Zhao H, Zheng J, Hu F, Fang B (2014) Liquid chromatography-tandem mass spectrometry method for toxicokinetics, tissue distribution, and excretion studies of T-2 toxin and its major metabolites in pigs. J Chromatogr B Anal Technol Biomed Life Sci 958:75–82
Petrakova AV, Urusov AE, Zherdev AV, Liu L, Xu C, Dzantieva BB (2017) Application of magnetite nanoparticles for the development of highly sensitive immunochromatographic test systems for mycotoxin detection. APPL BIOCHEM MICRO+ 53:470–475
Li CL, Wen K, Mi TJ, Zhang XY, Zhang HY, Zhang SX, Shen JZ, Wang ZH (2016) A universal multi-wavelength fluorescence polarization immunoassay for multiplexed detection of mycotoxins in maize. Biosens Bioelectron 79:258–265
Gao X, Cao WY, Chen MM, Xiong HY, Zhang XH, Wang SF (2014) A high sensitivity electrochemical sensor based on Fe3+-ion molecularly imprinted film for the detection of T-2 toxin. Electroanalysis 26:2739–2746
Rodriguez-Carrasco Y, Font G, Molto JC, Berrada H (2014) Quantitative determination of trichothecenes in breadsticks by gas chromatography-triple quadrupole tandem mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31(8):1422–1430
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2017YFC1200903, 2017YFF0108403, 2017YFC1601205), the National Natural Science Foundation of China (No.81703229, 31701711).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare no financial and personal relationships with other people or organizations that can inappropriately influence our work. No professional or other personal interest of any nature or kind in any product, service and/or companycould be construed as influencing the position presented in or the review of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1612 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Wang, Y., Yuan, S. et al. Competitive fluorometric assay for the food toxin T-2 by using DNA-modified silver nanoclusters, aptamer-modified magnetic beads, and exponential isothermal amplification. Microchim Acta 186, 219 (2019). https://doi.org/10.1007/s00604-019-3322-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3322-z