Abstract
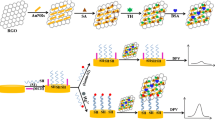
An impedimetric method is described for ultrasensitive analysis of mercury(II). It is based on thymine-Hg(II)-thymine interaction which causes the disintegration of multiple-sandwich structured DNA chains. DNA strands were selected that are partially complementary to the T-rich Hg(II)-specific oligonucleotides (MSO). They were immobilized on a gold electrode via Au-S interaction. Next, the MSO and the bridging strands (BS) that can connect adjacent MSOs were alternately attached through layer-by-layer hybridization. Thus, a multiple-sandwich structured interface in created that carries numerous MSOs. This leads to a change-transfer resistance (Rct) values of the electrode-electrolyte interface at faradic electrochemical impedance spectroscopy measurements in the presence of the hexacyanoferrate(II)/(III) redox probe at 0.2 V (vs. Ag/AgCl). If Hg(II) is added to the solution, the MSOs selectively interact with Hg(II) to produce T-Hg(II)-T structures. Hence, the multiple-sandwich hybridization chains become disintegrated, and this causes a decrease in resistivity. The effect can be used to quantify Hg(II) over an analytical range that extends over four orders of magnitude (1 fM to 10 pM), and it has a 0.16 fM limit of detection under optimal conditions.

An electrochemical sensor for femtomolar level detection of Hg2+ is realized on the basis of thymine-Hg2+-thymine interaction which causes disintegration of multiple sandwich DNA hybridization strands.




Similar content being viewed by others
References
Bhan A, Sarkar N (2011) Mercury in the environment: effect on health and reproduction. Rev Environ Health 20:39–56
Li P, Du B, Chan H, Feng X, Li B (2018) Mercury bioaccumulation and its toxic effects in rats fed with methylmercury polluted rice. Sci Total Environ 633:93–99
Xu X, Nie S, Ding H, Hou F (2018) Environmental pollution and kidney diseases. Nat Rev Nephrol 14:313–324
Cariccio V, Samà A, Bramanti P, Mazzon E (2018) Mercury involvement in neuronal damage and in neurodegenerative diseases. Biol Trace Elem Res:1–16
Clarkson T, Magos L, Myers G (2003) The toxicology of mercury-current exposures and clinical manifestations. N Engl J Med 349:1731–1737
Yavuz E, Tokalıoğlu Ş, Patat Ş (2018) Magnetic dispersive solid phase extraction with graphene/ZnFe2O4 nanocomposite adsorbent for the sensitive determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry. Microchem J 142:85–93
Bloxham M, Hill S, Worsfold P (1996) Determination of mercury in filtered sea-water by flow injection with on-line oxidation and atomic fluorescence spectrometric detection. J Anal At Spectrom 11:511–514
Yue Q, Shen T, Wang J, Wang L, Xu S, Li H, Liu J (2013) A reusable biosensor for detecting mercury(II) at the subpicomolar level based on “turn-on” resonance light scattering. Chem Commun 49:1750–1752
Huang F, Jiang S, Chen Y, Sahayam A (2018) Chemical vapor generation sample introduction for the determination of As, Cd, Sb, Hg, and Pb in nail polish by inductively coupled plasma mass spectrometry. Spectrochim Acta B 140:84–88
Lee C, Kang S, Oh J, Eom M, Oh J, Kim M, Lee W, Hong S, Han M (2017) A colorimetric and fluorescent chemosensor for detection of Hg2+ using counterion exchange of cationic polydiacetylene. Tetrahedron Lett 58:4340–4343
Shyamal M, Maity S, Maity A, Maity R, Roy S, Misra A (2018) Aggregation induced emission based “turn-off” fluorescent chemosensor for selective and swift sensing of mercury (II) ions in water. Sensor Actuat B-Chem 263:347–359
Suherman A, Ngamchuea K, Tanner E, Sokolov S, Alex L, Holter J, Young N, Compton R (2017) Electrochemical detection of ultratrace (picomolar) levels of Hg2+ using a silver nanoparticle-modified glassy carbon electrode. Anal Chem 89:7166–7173
Alizadeh T, Hamidi N, Ganjali MR, Rafiei F (2018) Determination of subnanomolar levels of mercury(II) by using a graphite paste electrode modified with MWCNTs and Hg(II) -imprinted polymer nanoparticles. Microchim Acta 185:16
Wang H, Liu Y, Liu G (2018) Electrochemical biosensor using DNA embedded phosphorothioate modified RNA for mercury ion determination. ACS Sens 3:624–631
Zahid A, Shah A, Iftikhar F, Shah A, Qureshi R (2017) Development of surfactant based electrochemical sensor for the trace level detection of mercury. Electrochim Acta 235:72–78
Chun HJ, Kim S, Han YD, Kim DW, Kim KR, Kim HS, Kim JH, Yoon HC (2018) Water-soluble mercury ion sensing based on the thymine-Hg2+-thymine base pair using retroreflective Janus particle as an optical signaling probe. Biosens Bioelectron 104:138–144
Amiri S, Ahmadi R, Salimi A, Navaee A, Qaddare SH, Aminic MK (2018) Ultrasensitive and highly selective FRET aptasensor for Hg2+ measurement in fish samples using carbon dots/AuNPs as donor/acceptor platform. New J Chem 42:16027–16035
Li MK, Hu LY, Niu CG, Huang DW, Zeng GM (2018) A fluorescent DNA based probe for Hg(II) based on thymine-Hg(II)-thymine interaction and enrichment via magnetized graphene oxide. Microchim Acta 185:207
Babamiria B, Salimia A, Hallaj R (2018) Switchable electrochemiluminescence aptasensor coupled with resonance energy transfer for selective attomolar detection of Hg2+ via CdTe@CdS/dendrimer probe and Au nanoparticle quencher. Biosens Bioelectron 102:328–335
Vilian ATE, Shahzad A, Chung JY, Choe SR, Kim WS, Huh YS, Yu T, Han YK (2017) Square voltammetric sensing of mercury at very low working potential by using oligomer-functionalized Ag@Au core-shell nanoparticles. Microchim Acta 184:3547–3556
He LL, Cheng L, Lin Y, Cui HF, Hong N, Peng H, Kong DR, Chen CD, Zhang J, Wei GB, Fan HA (2018) Sensitive biosensor for mercury ions detection based on hairpin hindrance by thymine-Hg(II)-thymine structure. J Electroanal Chem 814:161–167
Xu AG, Chao L, Xiao HB, Sui YY, Liu J, Xie QJ, Yao SZ (2018) Ultrasensitive electrochemical sensing of Hg2+ based on thymine-Hg2+-thymine interaction and signal amplification of alkaline phosphatase catalyzed silver deposition. Biosens Bioelectron 104:95–101
Gan XR, Zhao HM, Chen S, Quan X (2015) Electrochemical DNA sensor for specific detection of picomolar Hg(II) based on exonuclease III-assisted recycling signal amplification. Analyst 140:2029–2036
Xie M, Zhang K, Zhu F, Wu H, Zou P (2017) Strategy for the detection of mercury ions by using exonuclease III-aided target recycling. RSC Adv 7:50420–50424
Wu SH, Zhang B, Wang FF, Mi ZZ, Sun JJ (2018) Heating enhanced sensitive and selective electrochemical detection of Hg2+ based on T-Hg2+-T structure and exonuclease III-assisted target recycling amplification strategy at heated gold disk electrode. Biosens Bioelectron 104:145–151
Shah P, Zhu X, Zhang X, He J, Li C (2016) Microelectromechanical system-based sensing arrays for comparative in vitro nanotoxicity assessment at single cell and small cell-population using electrochemical impedance spectroscopy. ACS Appl Mater Inter 8:5804–5812
Ward A, Hannah A, Kendrick S, Tucker N, MacGregor G, Connolly P (2018) Identification and characterisation of staphylococcus aureus on low cost screen printed carbon electrodes using impedance spectroscopy. Biosens Bioelectron 110:65–70
Li S, Qiu W, Zhang X, Ni J, Gao F, Wang Q (2015) A high-performance DNA biosensor based on the assembly of gold nanoparticles on the terminal of hairpin-structured probe DNA. Sensors Actuators B Chem 223:861–867
Guo L, Yin N, Nie D, Fu F, Chen G (2011) An ultrasensitive electrochemical sensor for the mercuric ion via controlled assembly of SWCNTs. Chem Commun 47:10665–10667
Wang G, Huang H, Zhang X, Wang L (2012) Electrically contacted enzyme based on dual hairpin DNA structure and its application for amplified detection of Hg2+. Biosens Bioelectron 35:108–114
Mor-Piperberg G, Tel-Vered R, Elbaz J, Willner I (2010) Nanoengineered electrically contacted enzymes on DNA scaffolds: functional assemblies for the selective analysis of Hg2+ ions. J Am Chem Soc 132:6878–6879
Cai W, Xie S, Zhang J, Tang D, Tang Y (2017) An electrochemical impedance biosensor for Hg2+ detection based on DNA hydrogel by coupling with DNAzyme-assisted target recycling and hybridization chain reaction. Biosens Bioelectron 98:466–472
Gan X, Zhao H, Chen S, Quan X (2015) Electrochemical DNA sensor for specific detection of picomolar Hg(II) based on exonuclease III-assisted recycling signal amplification. Analyst 6:1–21
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21802064, 21575027), Natural Science Foundation of Fujian Province (Nos. 2018 J01435, 2017 J01419), and Foundation of Key Laboratory of Sensor Analysis of Tumor Marker from Ministry of Education, Qingdao University of Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 607 kb)
Rights and permissions
About this article
Cite this article
Gao, F., Zhang, T., Chu, Y. et al. Ultrasensitive impedimetric mercury(II) sensor based on thymine-Hg(II)-thymine interaction and subsequent disintegration of multiple sandwich-structured DNA chains. Microchim Acta 185, 555 (2018). https://doi.org/10.1007/s00604-018-3097-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3097-7