Abstract
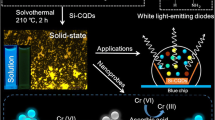
A fluorometric quenching assay is described for the determination of chromate(VI) by using a nanocomposite probe consisting of carbon quantum dots (CQDs) and phosphotungstic acid (HPW). The stable nanoprobe was synthesized via hydrothermal carbonization of glucose in the presence of HPW. HPW promotes the dehydration and carbonization and acts as an “electronic receptor”. It blocks the radiative electron/hole recombination in the CQDs and leads to a product whose fluorescence (with excitation/emission peaks at 360/463 nm) is quenched. The CQD/HPW was characterized by transmission electron microscopy, FT-IR spectroscopy, X-ray photoelectron spectroscopy, Raman spectroscopy, UV-vis absorption and fluorescence spectroscopy to characterize their surface morphology, functional groups and elemental composition, crystal structure and optical properties. The nanocomposite is nearly mono-disperse with an average particle diameter of 1.7 nm, and displays excitation wavelength-dependent and pH-dependent photoluminescence. Fluorescence drops on addition of chromate(VI) due to an inner filter effect. The ability of receiving electron for HPW can hinder the electron transfer from CQD/HPW to other metal ions, so the nanocomposite showed excellent selectivity towards chromate(VI). Fluorescence drops linearly with the concentration of chromate(VI) in the range from 2 to 80 μM, with a limit of detection of 0.16 μM.

Hydrothermal carbonization preparation of carbon quantum dots and phosphotungstic acid nanocomposite probe for fluorometric determination of chromate(VI) based on inner filter effect.





Similar content being viewed by others
References
Costa M (1997) Toxicity and carcinogenicity of chromate(VI) in animal models and humans. Crit Rev Toxicol 27(5):431–442. https://doi.org/10.3109/10408449709078442
Silva AS, Brandao GC, Matos GD, Ferreira SL (2015) Direct determination of chromate in infant formulas employing high-resolution continuum source electrothermal atomic absorption spectrometry and solid sample analysis. Talanta 144:39–43. https://doi.org/10.1016/j.talanta.2015.05.046
Islam A, Ahmad H, Zaidi N, Kumar S (2014) Graphene oxide sheets immobilized polystyrene for column preconcentration and sensitive determination of lead by flame atomic absorption spectrometry. ACS Appl Mater Interfaces 6(15):13257–13265. https://doi.org/10.1021/am5031215
Jin W, Wu GS, Chen AC (2014) Sensitive and selective electrochemical detection of chromate(VI) based on gold nanoparticle-decorated titania nanotube arrays. Analyst 139(1):235–241. https://doi.org/10.1039/C3AN01614E
Guo JF, Huo DQ, Mei Y, Hou CJ, Li JJ, Fa HB, Luo HB, Yang P (2016) Colorimetric detection of Cr (VI) based on the leaching of gold nanoparticles using a paper-based sensor. Talanta 161:819–825. https://doi.org/10.1016/j.talanta.2016.09.032
Wang ZJ, Wang XJ, Niu WP, Feng LH (2017) A specific fluorescence probe for chromate (VI) anions and its application. Sensors Actuators B Chem 244:727–731. https://doi.org/10.1016/j.snb.2017.01.067
Sui CX, Liu YF, Zhang WH, Li PA, Zhang D (2014) CdTe-CdSe nanocrystals capped with dimethylaminoethanethiol as ultrasensitive fluorescent probes for chromate(VI). Microchim Acta 181(3–4):347–353. https://doi.org/10.1007/s00604-013-1125-1
Zheng M, Xie ZG, Qu D, Li D, Du P, Jing XB, Sun ZC (2013) On-off-on fluorescent carbon dot nanosensor for recognition of chromate(VI) and ascorbic acid based on the inner filter effect. Appl Mater Interfaces 5(24):13242–13247. https://doi.org/10.1021/am4042355
Chen JC, Liu JH, Li JZ, Xu LQ, Qiao YJ (2017) One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J Colloid Interface Sci 485:167–174. https://doi.org/10.1016/j.jcis.2016.09.040
Xiao DL, Pan RF, Li SQ, He J, Qi M, Kong SM, Gu Y, Lin R, He H (2014) Porous carbon quantum dots: one step green synthesis via L-cysteine and applications in metal ion detection. RSC Adv 5(3):2039–2046. https://doi.org/10.1039/C4RA11179F
Zhang HQ, Huang YH, Hu ZB, Tong CQ, Zhang ZS, Hu SR (2017) Carbon dots codoped with nitrogen and sulfur are viable fluorescent probes for chromate(VI). Microchim Acta 184(5):1547–1553. https://doi.org/10.1007/s00604-017-2132-4
Zhang L, Zhang ZY, Liang RP, Li YH, Qiu JD (2014) Boron-doped graphene quantum dots for selective glucose sensing based on the "abnormal" aggregation-induced photoluminescence enhancement. Anal Chem 86(9):4423–4430. https://doi.org/10.1021/ac500289c
Zhu AW, Luo ZQ, Ding CQ, Li B, Zhou S, Wang R, Tian Y (2014) A two-photon "turn-on" fluorescent probe based on carbon nanodots for imaging and selective biosensing of hydrogen sulfide in live cells and tissues. Analyst 139(8):1945–1952. https://doi.org/10.1039/c3an02086j
Shen C, Sun YP, Wang J, Lu Y (2014) Facile route to highly photoluminescent carbon nanodots for ion detection, pH sensors and bioimaging. Nanoscale 6(15):9139–9147. https://doi.org/10.1039/c4nr02154a
Wang CX, Xu ZZ, Cheng H, Lin HH, Humphrey MG, Zhang C (2015) A hydrothermal route to water-stable luminescent carbon dots as nanosensors for pH and temperature. Carbon 82:87–95. https://doi.org/10.1016/j.carbon.2014.10.035
Ding H, Yu SB, Wei JS, Xiong HM (2016) Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 10(1):484–491. https://doi.org/10.1021/acsnano.5b05406
Mehta VN, Jha S, Kailasa SK (2014) One-pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells. Mate Sci Eng C Mater Biol Appl 38:20–27. https://doi.org/10.1016/j.msec.2014.01.038
Zhang H, Xie AJ, Shen YH, Qiu LG, Tian XY (2012) Layer-by-layer inkjet printing of fabricating reduced graphene-polyoxometalate composite film for chemical sensors. Phys Chem Chem Phys Pccp 14(37):12757. https://doi.org/10.1039/C2CP41561E
He XD, Li HT, Liu Y, Huang H, Kang ZH, Lee ST (2011) Water soluble carbon nanoparticles: hydrothermal synthesis and excellent photoluminescence properties. Colloids Surf B Biointerfaces 87(2):326–332. https://doi.org/10.1016/j.colsurfb.2011.05.036
Zhou D, Han BH (2010) Graphene-based Nanoporous materials assembled by mediation of Polyoxometalate nanoparticles. Adv Funct Mater 20(16):2717–2727. https://doi.org/10.1002/adfm.200902323
Pan DY, Guo L, Zhang JC, Xi C, Xue Q, Huang H, Li JH, Zhang ZW, Yu WJ, Chen ZW, Li Z, Wu MH (2012) Cutting sp(2) clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J Mater Chem 22(8):3314–3318. https://doi.org/10.1039/c2jm16005f
Zhu CZ, Zhai JF, Dong SJ (2012) Bifunctional fluorescent carbon nanodots: green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem Commun 48(75):9367–9369. https://doi.org/10.1039/c2cc33844k
Wu Q, Li W, Wu P, Li J, Liu SX, Jin CD, Zhan XX (2015) Effect of reaction temperature on properties of carbon nanodots and their visible-light photocatalytic degradation of tetracyline. RSC Adv 5(92):75711–75721. https://doi.org/10.1039/c5ra16080d
Ding H, Ji Y, Wei JS, Gao QY, Zhou ZY, Xiong HM (2017) Facile synthesis of red-emitting carbon dots from pulp-free lemon juice for bioimaging. J Mat Chem B 5(26):5272–5277. https://doi.org/10.1039/C7TB01130J
Zhu SJ, Song YB, Zhao XH, Shao JR, Zhang JH, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8(2):355–381. https://doi.org/10.1007/s12274-014-0644-3
Sahu S, Behera B, Maiti TK, Mohapatra S (2012) Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun 48(70):8835–8837. https://doi.org/10.1039/c2cc33796g
Cai F, Liu XD, Liu S, Liu H, Huang YM (2014) A simple one-pot synthesis of highly fluorescent nitrogen-doped graphene quantum dots for the detection of Cr(VI) in aqueous media. RSC Adv 4(94):52016–52022. https://doi.org/10.1039/C4RA09320H
Zhang HY, Wang Y, Xiao S, Wang H, Wang JH, Feng L (2017) Rapid detection of Cr(VI) ions based on cobalt(II)-doped carbon dots. Biosens Bioelectron 87:46–52. https://doi.org/10.1016/j.bios.2016.08.010
Gong XJ, Liu Y, Yang ZH, Shuang SM, Zhang ZY, Dong C (2017) An “on-off-on” fluorescent nanoprobe for recognition of chromium(VI) and ascorbic acid based on phosphorus/nitrogen dual-doped carbon quantum dot. Anal Chim Acta 968:85–96. https://doi.org/10.1016/j.aca.2017.02.038
Guo YM, Chen YZ, Cao FP, Wang LJ, Wang Z, Leng YM (2017) Hydrothermal synthesis of nitrogen and boron doped carbon quantum dots with yellow-green emission for sensing Cr(VI), anti-counterfeiting and cell imaging. RSC Adv 7(76):48386–48393. https://doi.org/10.1039/C7RA09785A
Zhu LJ, Peng X, Li HT, Zhang YY, Yao SZ (2017) On-off-on fluorescent silicon nanoparticles for recognition of chromium(VI) and hydrogen sulfide based on the inner filter effect. Sensors Actuators B Chem 238:196–203. https://doi.org/10.1016/j.snb.2016.07.029
Cui ML, Wang C, Yang DP, Song QJ (2018) Fluorescent iridium nanoclusters for selective determination of chromium(VI). Microchim Acta 185(1):8. https://doi.org/10.1007/s00604-017-2553-0
Zhang LJ, Xu CL, Li BX (2009) Simple and sensitive detection method for chromium(VI) in water using glutathione-capped CdTe quantum dots as fluorescent probes. Microchim Acta 166(1–2):61–68. https://doi.org/10.1007/s00604-009-0164-0
Xin JW, Zhang FQ, Gao YX, Feng YY, Chen SG, Wu AG (2012) A rapid colorimetric detection method of trace Cr (VI) based on the redox etching of ag core -au shell nanoparticles at room temperature. Talanta 101(101):122–127. https://doi.org/10.1016/j.talanta.2012.09.009
Ravindran A, Elavarasi M, Prathna TC, Raichur AM, Chandrasekaran N, Mukherjee A (2012) Selective colorimetric detection of nanomolar Cr (VI) in aqueous solutions using unmodified silver nanoparticles. Sensors Actuators B Chem 166-167(6):365–371. https://doi.org/10.1016/j.snb.2012.02.073
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 31570567, 31500467), Fundamental Research Funds for the Central Universities of China (Nos. 2572017ET02, 2572018AB02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 4.25 mb)
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, Z., Li, W. et al. A nanocomposite probe consisting of carbon quantum dots and phosphotungstic acid for fluorometric determination of chromate(VI) with improved selectivity. Microchim Acta 185, 470 (2018). https://doi.org/10.1007/s00604-018-2993-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2993-1