Abstract
The article describes a reusable biosensor for Pb(II) ions. A duplex DNA with a terminal amino group and containing a G-quadruplex (G4) aptamer was covalently conjugated to single walled carbon nanotubes on a field effect transistor (FET). The detection scheme is based on the despiralization of the DNA duplex because Pb(II) can induce the G4 aptamer to form a stabilizing G4/Pb(II) complex. This structural change affects the electrical conductivity of SWNTs which serves as the analytical signal. The biosensor was characterized via scanning electron microscopy, Raman, UV-vis, and voltage-current profiles. Under optimized conditions, the relative resistance at 0.02 V increases linearly with the logarithm of the Pb(II) concentration in the range from 1 ng·L−1 to 100 μg·L−1, and the limit of detection is 0.39 ng·L−1. Compared to other sensors, this oner demonstrates superior simplicity, sensitivity, and selectivity even in mixtures of heavy metal ions. It was applied to the determination of Pb(II) in (spiked) water and soil samples and gave good results.

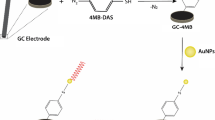
Schematic of the fabrication a biosensor for Pb(II). It is making use of an SWNT-based FET, G4-DNA and complementary DNA with an amino group. Pb(II) can despiralize the DNA duplex to form a G-quadruplex which affects the electrical conductivity of SWNTs. After each detection, the single complementary strand DNA can rebind the G4-DNA, which makes the biosensor reusable.







Similar content being viewed by others
References
Haefliger P, Mathieu-Nolf M, Lociciro S, Ndiaye C, Coly M, Diouf A, Faye AL, Sow A, Tempowski J, Pronczuk J (2009) Mass lead intoxication from informal used lead-acid battery recycling in Dakar, Senegal. Environ Health Perspect 117:1535
Chen K, Huang L, Yan BZ, Li HB, Sun H, Bi J (2014) Effect of lead pollution control on environmental and childhood blood lead level in Nantong, China: an interventional study. Environ Sci Technol 48:12930–12936
Walraven N, Bakker M, van Os B, Klaver G, Middelburg JJ, Davies G (2016) Pollution and oral bioaccessibility of Pb in soils of villages and cities with a long habitation history. Int J Env Res Pub He 13
He QW, Miller EW, Wong AP, Chang CJ (2006) A selective fluorescent sensor for detecting lead in living cells. J Am Chem Soc 128:9316–9317
Li T, Shi LL, Wang EK, Dong SJ (2009) Silver-ion-mediated DNAzyme switch for the ultrasensitive and selective colorimetric detection of aqueous Ag+ and cysteine. Chem-Eur J 15:3347–3350
Yu Y, Li YX, Li B, Shen ZY, Stenstrom MK (2016) Metal enrichment and lead isotope analysis for source apportionment in the urban dust and rural surface soil. Environ Pollut 216:764–772
Ma W (1996) Lead in mammals, environmental contaminants in wildlife: interpreting tissue concentrations. CRC Press, Boca Raton, pp 281–296
Pareja-Carrera J, Mateo R, Rodríguez-Estival J (2014) Lead (Pb) in sheep exposed to mining pollution: implications for animal and human health. Ecotox Environ Safe 108:210–216
Gourlaouen C, Parisel O (2007) Is an electronic shield at the molecular origin of lead poisoning? A computational modeling experiment. Angew Chem Ed 46:553–556
Bhattacharjee T, Bhattacharjee S, Choudhuri D (2016) Hepatotoxic and nephrotoxic effects of chronic low dose exposure to a mixture of heavy metals–lead, cadmium and arsenic. Int J Pharma Chem Biol Sci 6:39–47
Araujo J, Beelen A, Lewis L, Robinson G, DeLaurier C, Carbajal M, Ericsson B, Chin Y, Hipkins K, Kales S (2004) Lead poisoning associated with Ayurvedic medications—five states, 2000-2003, MMWR- Morbidand mortal w. 53: 582
Kernich CA (2001) Peripheral neuropathy. Neurologist 7:315–316
Duran C, Gundogdu A, Bulut VN, Soylak M, Elci L, Senturk HB, Tufekci M (2007) Solid-phase extraction of Mn(II), Co(II), Ni(II), Cu(II), Cd(II) and Pb(II) ions from environmental samples by flame atomic absorption spectrometry (FAAS). J Hazard Mater 146:347–355
Sorbo A, Turco AC, Di Gregorio M, Ciaralli L (2014) Development and validation of an analytical method for the determination of arsenic, cadmium and lead content in powdered infant formula by means of quadrupole inductively coupled plasma mass spectrometry. Food Control 44:159–165
Feist B, Mikula B (2014) Preconcentration of heavy metals on activated carbon and their determination in fruits by inductively coupled plasma optical emission spectrometry. Food Chem 147:302–306
Pushie MJ, Pickering IJ, Korbas M, Hackett MJ, George GN (2014) Elemental and chemically specific X-ray fluorescence imaging of biological systems. Chem Rev 114:8499–8541
Beltran B, Leal LO, Ferrer L, Cerda V (2015) Determination of lead by atomic fluorescence spectrometry using an automated extraction/pre-concentration flow system. J Anal Atom Spectrom 30:1072–1079
Li XY, Wu ZT, Zhou XD, Hu JM (2017) Colorimetric response of peptide modified gold nanoparticles: an original assay for ultrasensitive silver detection. Biosens Bioelectron 92:496–501
Xiao Y, Rowe AA, Plaxco KW, Heeger AJ (2007) Electrochemical detection of parts per billion lead via an electrode-bound DNAzyme assembly. Abstr Pap Am Chem S 233:449–449
Chung CH, Kim JH, Jung J, Chung BH (2013) Nuclease-resistant DNA aptamer on gold nanoparticles for the simultaneous detection of Pb2+ and Hg2+ in human serum. Biosens Bioelectron 41:827–832
Long F, Zhu A, Wang HC (2014) Optofluidics-based DNA structure-competitive aptasensor for rapid on-site detection of lead(II) in an aquatic environment. Anal Chim Acta 849:43–49
Rance GA, Marsh DH, Nicholas RJ, Khlobystov AN (2010) UV-vis absorption spectroscopy of carbon nanotubes: relationship between the pi-electron plasmon and nanotube diameter. Chem PhysLett 493:19–23
Bao Q, Zhang H, Yang JX, Wang S, Tang DY, Jose R, Ramakrishna S, Lim CT, Loh KP (2010) Graphene–polymer nanofiber membrane for ultrafast photonics. Adv Funct Mater 20:782–791
Guo S, Lin J, Penchev M, Yangel E, Ozkan M, Ozkan CS (2011) Label free DNA detection using large area Graphene-based FET biosensors. J Nanosci Nanotechno 11:5258–5263
Takeshima T, Sun L, Wang Y, Yamada Y, Nishi N, Yonezawa T, Fugetsu B (2014) Salmon milt DNA as a template for the mass production of Ag nanoparticles. Polym J 46:36
Jorio A, Fantini C, Dantas MSS, Pimenta MA, Souza AG, Samsonidze GG, Brar VW, Dresselhaus G, Dresselhaus MS, Swan AK, Unlu MS, Goldberg BB, Saito R (2002) Linewidth of the Raman features of individual single-wall carbon nanotubes. Phys Rev B 66(11):1–8
Byon HR, Choi HC (2006) Network single-walled carbon nanotube-field effect transistors (SWNT-FETs) with increased Schottky contact area for highly sensitive biosensor applications. J Am Chem Soc 128:2188–2189
Jiokeng SLZ, Dongmo LM, Ymele E, Ngameni E, Tonle IK (2017) Sensitive stripping voltammetry detection of Pb(II) at a glassy carbon electrode modified with an amino-functionalized attapulgite. Sensor Actuat B-Chem 242:1027–1034
Zidarič T, Jovanovski V, Menart E, Zorko M, Kolar M, Veber M, Hočevar S (2017) Multi-pulse galvanostatic preparation of nanostructured bismuth film electrode for trace metal detection. Sensor Actuat B-Chem 245:720–725
Xing H, Xu J, Zhu X, Duan X, Lu L, Wang W, Zhang Y, Yang T (2016) Highly sensitive simultaneous determination of cadmium (II), lead (II), copper (II), and mercury (II) ions on N-doped graphene modified electrode. Electroanal Chem 760:52–58
Taghdisi SM, Danesh NM, Lavaee P, Ramezani M, Abnous K (2016) An electrochemical aptasensor based on gold nanoparticles, thionine and hairpin structure of complementary strand of aptamer for ultrasensitive detection of lead. Sensor Actuat B-Chem 234:462–469
Zhou Y, Tang L, Zeng G, Zhang C, Xie X, Liu Y, Wang J, Tang J, Zhang Y, Deng Y (2016) Label free detection of lead using impedimetric sensor based on ordered mesoporous carbon–gold nanoparticles and DNAzyme catalytic beacons. Talanta 146:641–647
Wang C, Cui X, Li Y, Li H, Huang L, Bi J, Luo J, Ma LQ, Zhou W, Cao Y (2016) A label-free and portable graphene FET aptasensor for children blood lead detection. Sci Rep 6:21711
Kanyong P, Rawlinson S, Davis J (2016) Gold nanoparticle modified screen-printed carbon arrays for the simultaneous electrochemical analysis of lead and copper in tap water. Microchim Acta 183:2361–2368
Wang H, Zhao G, Zhang Z, Yi Y, Wang Z, Liu G (2017) A portable electrochemical workstation using disposable screen-printed carbon electrode decorated with multiwall carbon nanotube-ionic liquid and bismuth film for Cd (II) and Pb (II) determination. Int. J Electrochem Sci 12:4702–4713
Acknowledgements
This work was supported by Chinese National Natural Science Foundation (No. 31671578), National High Technology Research and Development Program of China (No.2013AA102302), the Fundamental Research Funds for the Central Universities (No. 2016 XD001) and the Shandong Provincial Natural Science Foundation of China (No. ZR2015CM016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing financial interests.
Electronic supplementary material
ESM 1
(DOCX 380 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Liu, Y. & Liu, G. Reusable resistive aptasensor for Pb(II) based on the Pb(II)-induced despiralization of a DNA duplex and formation of a G-quadruplex. Microchim Acta 185, 142 (2018). https://doi.org/10.1007/s00604-018-2682-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2682-0