Abstract
The authors are presenting an electrochemical aptasensor for tumor necrosis factor-alpha (TNF-α) detection that is aided by the use of magnetic nanoparticles (NPs) and two DNA probes. Firstly, magnetic NPs coated with gold NPs (Fe3O4@AuNP) are synthesized. Then, DNA probe 1 with a terminal thiol group is immobilized on the surface of Fe3O4@AuNP via gold-thiol chemistry. DNA probe 1 is then hybridized with DNA probe 2, which is labeled with Methylene Blue (MB). The composite of Fe3O4@AuNP-DNA duplex is formed as a result, which can be easily absorbed on a magnetized glassy carbon electrode. The electrochemical signal is obtained after reductant-mediated amplification. Since MB-labeled DNA probe 2 is the aptamer against TNF-α, it will be released in the presence of TNF-α. This process leaves Fe3O4@AuNP-DNA probe 1 on the surface of the electrode. Thus, the recorded electrochemical response decreases dramatically. The assay, best operated at a working voltage of −0.39 V (vs. SCE), has a linear response in the 10 pg mL−1 to 100 ng·mL−1 TNF-α concentration range, and the limit of detection is 10 pg mL−1. The sensing strategy is highly sensitive and selective, and has been successfully applied to real samples.

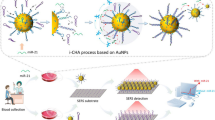
Schematic of the biosensor. The composite of Fe3O4@AuNP-DNA duplex is placed on a glassy carbon electrode. After interaction between TNF-α and its aptamer, DNA probe is released with the labeled electrochemical species. The decrease in electrochemical response serves as the analytical signal.






Similar content being viewed by others
References
Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou LL, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17:1049–1061
Crouse J, Kalinke U, Oxenius A (2015) Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol 15:231–242
Iwasaki A, Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16:343–353
Brestoff JR, Artis D (2015) Immune regulation of metabolic homeostasis in health and disease. Cell 161:146–160
Collnot EM, Ali H, Lehr CM (2012) Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release 161:235–246
Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A (2007) TNF-alpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci U S A 104:12843–12848
Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL (2011) TGF beta/TNF alpha-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res 71:4707–4719
Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271:665–668
Mazloum-Ardakani M, Hosseinzadeh L, Taleat Z (2014) Two kinds of electrochemical immunoassays for the tumor necrosis factor alpha in human serum using screen-printed graphite electrodes modified with poly(anthranilic acid). Microchim Acta 181:917–924
Oh BR, Huang NT, Chen WQ, Seo JH, Chen PY, Cornell TT, Shanley TP, Fu JP, Kurabayashi K (2014) Integrated nanoplasmonic sensing for cellular functional immunoanalysis using human blood. ACS Nano 8:2667–2676
Marulli E, Aloisi A, Di Giuseppe P, Rinaldi R (2016) Micro and nanotechnology for early diagnosis and detection of rheumatic diseases-molecular markers. BioChip J 10:189–197
Cheng WB, Yan W, Miao P (2017) TNF-α responsive DNA star trigon formation from four hairpin probes and the analytical application. Sci China-Chem 60:405–409
Qi M, Zhang Y, Cao CM, Zhang MX, Liu SH, Liu GZ (2016) Decoration of reduced graphene oxide nanosheets with aryldiazonium salts and gold nanoparticles toward a label-free amperometric immunosensor for detecting cytokine tumor necrosis factor-alpha in live cells. Anal Chem 88:9614–9621
Baydemir G, Bettazzi F, Palchetti I, Voccia D (2016) Strategies for the development of an electrochemical bioassay for TNF-alpha detection by using a non-immunoglobulin bioreceptor. Talanta 151:141–147
Man Y, Lv XF, Iqbal J, Peng G, Song D, Zhang CX, Deng YL (2015) Microchip based and immunochromatographic strip assays for the visual detection of interleukin-6 and of tumor necrosis factor alpha using gold nanoparticles as labels. Microchim Acta 182:597–604
Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A (2004) Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol 110:252–266
Mokrushina AV, Heim M, Karyakina EE, Kuhn A, Karyakin AA (2013) Enhanced hydrogen peroxide sensing based on Prussian blue modified macroporous microelectrodes. Electrochem Commun 29:78–80
Zhao J, Chen GF, Zhu L, Li GX (2011) Graphene quantum dots-based platform for the fabrication of electrochemical biosensors. Electrochem Commun 13:31–33
Miao P, Tang YG, Wang L (2017) DNA modified Fe3O4@au magnetic nanoparticles as selective probes for simultaneous detection of heavy metal ions. ACS Appl Mater Interfaces 9:3940–3947
Liu T, Yin J, Wang YH, Miao P (2016) Construction of a specific binding peptide based electrochemical approach for sensitive detection of Zn2+. J Electroanal Chem 783:304–307
Yu YJ, Zhang Q, Buscaglia J, Chang CC, Liu Y, Yang ZH, Guo YC, Wang YT, Levon K, Rafailovich M (2016) Quantitative real-time detection of carcinoembryonic antigen (CEA) from pancreatic cyst fluid using 3-D surface molecular imprinting. Analyst 141:4424–4431
Ning LM, Yang DW, Wang J, Miao P (2016) Highly sensitive detection of silver ions enabled by RecJ(f) exonuclease cleavage and reductant-mediated electrochemical amplification. Chem Electro Chem 3:1737–1740
Liu Y, Kwa T, Revzin A (2012) Simultaneous detection of cell-secreted TNF-alpha, and IFN-gamma using micropatterned aptamer-modified electrodes. Biomaterials 33:7347–7355
Liu Y, Zhou Q, Revzin A (2013) An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 138:4321–4326
Dai S, Feng CJ, Li W, Jiang W, Wang L (2014) Quantitative detection of tumor necrosis factor-alpha by single molecule counting based on a hybridization chain reaction. Biosens Bioelectron 60:180–184
Li T, Si ZZ, Hu LQ, Qi HZ, Yang MH (2012) Prussian blue-functionalized ceria nanoparticles as label for ultrasensitive detection of tumor necrosis factor-alpha. Sens Actuator B-Chem 171:1060–1065
Eletxigerra U, Martinez-Perdiguero J, Merino S, Villalonga R, Pingarron JM, Campuzano S (2014) Amperometric magnetoimmunoassay for the direct detection of tumor necrosis factor alpha biomarker in human serum. Anal Chim Acta 838:37–44
Sanchez-Tirado E, Salvo C, Gonzalez-Cortes A, Yanez-Sedeno P, Langa F, Pingarron JM (2017) Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double walled carbon nanotubes. Anal Chim Acta 959:66–73
Weng SH, Chen M, Zhao CF, Liu AL, Lin LQ, Liu QC, Lin JH, Lin XH (2013) Label-free electrochemical immunosensor based on K-3[Fe(CN)(6)] as signal for facile and sensitive determination of tumor necrosis factor-alpha. Sens Actuator B-Chem 184:1–7
Arya SK, Kongsuphol P, Park MK (2017) On-chip electrochemical immunoassay platform for specific protein biomarker estimation in undiluted serum using off-surface membrane matrix. Biosens Bioelectron 91:721–727
Arya SK, Estrela P (2017) Electrochemical immunosensor for tumor necrosis factor-alpha detection in undiluted serum. Methods 116:125–131
Pui TS, Kongsuphol P, Arya SK, Bansal T (2013) Detection of tumor necrosis factor (TNF-alpha) in cell culture medium with label free electrochemical impedance spectroscopy. Sens Actuator B-Chem 181:494–500
Sun ZF, Deng L, Gan H, Shen RJ, Yang MH, Zhang Y (2013) Sensitive immunosensor for tumor necrosis factor alpha based on dual signal amplification of ferrocene modified self-assembled peptide nanowire and glucose oxidase functionalized gold nanorod. Biosens Bioelectron 39:215–219
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 31400847) and the Science and Technology Program of Suzhou (Grant no. SYG201605).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Miao, P., Yang, D., Chen, X. et al. Voltammetric determination of tumor necrosis factor-α based on the use of an aptamer and magnetic nanoparticles loaded with gold nanoparticles. Microchim Acta 184, 3901–3907 (2017). https://doi.org/10.1007/s00604-017-2419-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2419-5