Abstract
The authors describe the use of gold nanoclusters (AuNCs) with a diameter of ~2 nm for fluorescent sensing of pH values in the range from 5 to 9. The AuNCs were synthesized in the presence of bovine serum albumin (BSA) which acts as both a reducing agent and capping agent. The resulting AuNCs were characterized in terms of size and surface chemistry using TEM and FTIR. The BSA-capped AuNCs display red luminescence, with excitation/emission peaks at 470/640 nm, which is strongly modulated by the pH indicator bromothymol blue (BTB). The effect depends mainly on an inner filter effect due to spectral overlap between the absorption BTB and the emission of the AuNCs. The pH nanosensor responds to pH values in the range from 5 to 9 which is the so-called physiological pH range. The method was applied to detect changes in the pH values that occur after the death of red blood cells. Such pH changes are considered as a potential forensic marker for estimating the time passed since death. The results show the BTB-BSA-AuNC system to be capable of detecting respective intracellular pH changes.

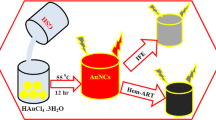
Schematic presentation of gold nanoclusters capped with bovine serum albumin whose red luminescence is modulated by the pH indicator bromothymol blue. The nanoclusters were applied as pH sensor in solution (5–9) and in blood cell imaging to estimate the time passed since death.







Similar content being viewed by others
References
Busa WB, Nuccitelli R (1984) Metabolic regulation via intracellular pH. Am J Physiol Regul Integr Comp Physiol 246:R409–R438
Putney LK, Barber DL (2003) Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem 278:44645–44469
Madshus IH (1988) Regulation of intracellular pH in eukaryotic cells. Biochem J 250:1–8
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W (2000) High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer res 60:916–921
Damaghi M, Wojtkowiak JW, Gillies RJ (2013) pH sensing and regulation in cancer. Front Physiol 4:370
Donaldson AE, Lamont IL (2013) Biochemistry changes that occur after death: potential markers for determining post-mortem interval. PLoS One 8:e82011
Hesse SJ, Ruijter GJ, Dijkema C, Visser J (2000) Measurement of intracellular (compartmental) pH by 31P NMR in Aspergillus niger. J Biotechnol 77:5–15
Orgovan G, Noszal B (2011) Electrodeless, accurate pH determination in highly basic media using a new set of (1)H NMR pH indicators. J Pharm Biomed Anal 54:958–964
Li Y, Wang Y, Yang S, Zhao Y, Yuan L, Zheng J, Yang R (2015) Hemicyanine-based high resolution ratiometric near-infrared fluorescent probe for monitoring pH changes in vivo. Anal Chem 87:2495–2503
Zhou X, Su F, Lu H, Senechal-Willis P, Tian Y, Johnson RH, Meldrum DR (2012) An FRET-based ratiometric chemosensor for in vitro cellular fluorescence analyses of pH. Biomaterials 33:171–180
Bizzarri R, Serresi M, Luin S, Beltram F (2009) Green fluorescent protein based pH indicators for in vivo use: a review. Anal Bioanal Chem 393:1107–1122
Keith BM, Bernard L, Sabahudin H, Geoff S, John HT (2008) Assessment of cytotoxicity of quantum dots andGold nanoparticles using cell-based ImpedanceSpectroscopy. Anal Chem 80:5487–5493
Knopp D, Tang D, Niessner R (2009) Review: bioanalytical applications of biomolecule-functionalized nanometer-sized doped silica particles. Anal Chim Acta 647:14–30
Sun LN, Peng H, Stich MI, Achatz D, Wolfbeis OS (2009) pH sensor based on upconverting luminescent lanthanide nanorods. Chem Commun (Camb) 33:5000–5002
Chen LY, Wang CW, Yuan Z, Chang HT (2015) Fluorescent gold nanoclusters: recent advances in sensing and imaging. Anal Chem 87:216–229
Khandelia R, Bhandari S, Pan UN, Ghosh SS, Chattopadhyay A (2015) Gold Nanocluster embedded albumin nanoparticles for two-photon imaging of cancer cells accompanying drug delivery. Small 11:4075–4081
Lee D, Donkers RL, Wang G, Harper AS, Murray RW (2004) Electrochemistry and optical absorbance and luminescence of molecule-like Au38 nanoparticles. J am Chem Soc 126:6193–6199
Negishi Y, Nobusada K, Tsukuda T (2005) Glutathione-protected gold clusters revisited: bridging the gap between gold(I)-thiolate complexes and thiolate-protected gold nanocrystals. J am Chem Soc 127:5261–5270
Xu Y, Sherwood J, Qin Y, Crowley D, Bonizzoni M, Bao Y (2014) The role of protein characteristics in the formation and fluorescence of au nanoclusters. Nano 6:1515–1524
Wang M, Mei Q, Zhang K, Zhang Z (2012) Protein-gold nanoclusters for identification of amino acids by metal ions modulated ratiometric fluorescence. Analyst 137:1618–1623
Lin CAJ, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, Li JK, Shen JL, Wang HH, Yeh HI, Parak, WJ, Chang WH (2009) Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 3:395–401
Xie J, Zheng Y, Ying JY (2010) Highly selective and ultrasensitive detection of hg(2+) based on fluorescence quenching of au nanoclusters by hg(2+) -au(+) interactions. Chem Commun (Camb) 46:961–963
Xie J, Zheng Y, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J am Chem Soc 131:888–889
Ding C, Tian Y (2014) Gold nanocluster-based fluorescence biosensor for targeted imaging in cancer cells and ratiometric determination of intracellular pH. Biosens Bioelectron 65C:183–190
Deng HH, Wu GW, Zou ZQ, Peng HP, Liu AL, Lin XH, Xia XH, Chen W (2015) pH-sensitive gold nanoclusters: preparation and analytical applications for urea, urease, and urease inhibitor detection. Chem Commun (Camb) 51:7847–7850
Ali R, Saleh SM, Meier RJ, Azab HA, Abdelgawad I, Wolfbeis OS (2010) Upconverting nanoparticle based optical sensor for carbon dioxide. Sens Actuators B Chem 150:126–131
Vidal E, Palomeque ME, Lista AG, Fernandez Band BS (2003) Flow injection analysis: Rayleigh light scattering technique for total protein determination. Anal Bioanal Chem 376:38–41
Zhou XC, Yan CQ, Yan CN (2010) Study on the interaction of bromothymol blue and bovine serum albumin[J]. Chemical Research and Application 1:003
Lin CA, Lee CH, Hsieh JT, Wang HH, Li JK, Shen JL, Chan WH, Yeh HI, Chang WH (2009) Review: synthesis of fluorescent metallic nanoclusters toward biomedical application: recent progress and present challenges. J med Biol Eng 29:276–283
Chakraborty D, Rajan G, Isaac R (2015) A splendid blend of nanotechnology and forensic Science. Journal of Nanotechnology in Engineering and Medicine 6:010801–010806
Chang HC, Ho JA (2015) Gold Nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal Chem 87:10362–10367
Baumann D, Hofmann D, Nullmeier S, Panther P, Dietze C, Musyanovych A, Ritz S, Landfester K, Mailänder V (2013) Complex encounters: nanoparticles in whole blood and their uptake into different types of white blood cells. Nanomedicine 8:699–713
Hsu NY, Lin YW (2016) Microwave-assisted synthesis of bovine serum albumin–gold nanoclusters and their fluorescence-quenched sensing of Hg2+ ions. New J Chem 40:1155–1161
Wang XL, Jie YA, Kui JI (2008) Electrochemical study on the interaction of protein with Bromothymol blue and its analytical application. Chem Res Chin Univ 24:701–706
Silverstein RM, Webster FX, Kiemle DJ (2005) Spectrometric identification of organic compounds. Wiley, New Delhi, pp 107–108
Donaldson AE, Lamont IL (2014) Estimation of post-mortem interval using biochemical markers. Aust J Forensic Sci 46:8–26
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 341 kb)
Rights and permissions
About this article
Cite this article
Ali, R., Saleh, S.M. & Aly, S.M. Fluorescent gold nanoclusters as pH sensors for the pH 5 to 9 range and for imaging of blood cell pH values. Microchim Acta 184, 3309–3315 (2017). https://doi.org/10.1007/s00604-017-2352-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2352-7