Abstract
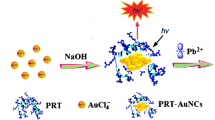
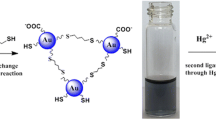
The authors describe a strategy for fluorometric determination of lead(II) that is based on the suppression of the surface energy transfer that occurs between acridine orange and gold nanoparticles (AuNPs). As a result, the fluorescence of the system is recovered. Under optimized conditions, the enhancement of fluorescence intensity is related to the concentration of lead(II) in the 44 nM to 4.8 μM range, with a detection limit of 13 nM. The relative standard deviations for 11 determinations at concentrations of 0.386 μM, 1.93 μM and 2.89 μM are 1.02 %, 1.06 % and 1.75 %, respectively. This result suggests that the method can potentially be used to monitor the level of lead(II) in environmental samples.

Lead(II) ion is found to bind to citrate capped gold nanoparticles (AuNPs) which prevents binding of acridine orange (AO) to the AuNPs. As a result, the surface energy transfer from AO to AuNPs cannot occur and fluorescence is restored. This effect was used to quantify lead(II) ion.


Similar content being viewed by others
References
Lu L, Cheng H, Liu X, Xie J, Li Q, Zhou T (2015) Assessment of regional human health risks from lead contamination in Yunnan province, southwestern China. PLoS One 10:e0119562
Monnot AD, Christian WV, Abramson MM, Follansbee MH (2015) An exposure and health risk assessment of lead (Pb) in lipstick. Food Chem Toxicol 80:253–260
Cao S, Duan X, Zhao X, Wang B, Ma J, Fan D, Sun C, He B, Wei F, Jiang G (2015) Health risk assessment of various metal(loid)s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ Pollut 200:16–23
Zhang D, Yin L, Meng Z, Yu A, Guo L, Wang H (2014) A sensitive fluorescence anisotropy method for detection of lead (II) ion by a G-quadruplex-inducible DNA aptamer. Anal Chim Acta 812:161–167
Tang S, Tong P, Li H, Tang J, Zhang L (2013) Ultrasensitive electrochemical detection of Pb2+ based on rolling circle amplification and quantum dots tagging. Biosens Bioelectron 42:608–611
Shokri M, Beiraghi A, Seidi S (2015) In situ emulsification microextraction using a dicationic ionic liquid followed by magnetic assisted physisorption for determination of lead prior to micro-sampling flame atomic absorption spectrometry. Anal Chim Acta 889:123–129
Wang Y, Chen H, Tang J, Ye G, Ge H, Hu X (2015) Preparation of magnetic metal organic frameworks adsorbent modified with mercapto groups for the extraction and analysis of lead in food samples by flame atomic absorption spectrometry. Food Chem 181:191–197
Sharafi K, Fattahi N, Pirsaheb M, Yarmohamadi H, Fazlzadeh Davil M (2015) Trace determination of lead in lipsticks and hair dyes using microwave-assisted dispersive liquid-liquid microextraction and graphite furnace atomic absorption spectrometry. Int J Cosmet Sci 37:489–495
Rello L, Aramendia M, Belarra MA, Resano M (2015) Lead screening in DBS by solid sampling high-resolution continuum source graphite furnace atomic absorption spectrometry: application to newborns and pregnant women. Bioanalysis 7:2057–2070
Giakisikli G, Ayala Quezada A, Tanaka J, Anthemidis AN, Murakami H, Teshima N, Sakai T (2015) Automatic on-line solid-phase extraction-electrothermal atomic absorption spectrometry exploiting sequential injection analysis for trace vanadium, cadmium and lead determination in human urine samples. Anal Sci 31:383–389
Demirtas I, Bakirdere S, Ataman OY (2015) Lead determination at ng/mL level by flame atomic absorption spectrometry using a tantalum coated slotted quartz tube atom trap. Talanta 138:218–224
Aghamohammadi M, Faraji M, Shahdousti P, Kalhor H, Saleh A (2015) Trace determination of lead, chromium and cadmium in herbal medicines using ultrasound-assisted emulsification microextraction combined with graphite furnace atomic absorption spectrometry. Phytochem Anal 26:209–214
Lopez-Garcia I, Vicente-Martinez Y, Hernandez-Cordoba M (2014) Determination of cadmium and lead in edible oils by electrothermal atomic absorption spectrometry after reverse dispersive liquid-liquid microextraction. Talanta 124:106–110
Soares AR, Nascentes CC (2013) Development of a simple method for the determination of lead in lipstick using alkaline solubilization and graphite furnace atomic absorption spectrometry. Talanta 105:272–277
Uemoto M, Nagaoka M, Fujinuma H (2009) Interlaboratory testing for the determination of trace amounts of tin and lead in magnesium and magnesium alloys by inductively coupled plasma atomic emission spectrometry. Anal Sci 25:717–721
Xu Y, Zhou J, Wang G, Zhou J, Tao G (2007) Determination of trace amounts of lead, arsenic, nickel and cobalt in high-purity iron oxide pigment by inductively coupled plasma atomic emission spectrometry after iron matrix removal with extractant-contained resin. Anal Chim Acta 584:204–209
Ganjali MR, Babaei LH, Badiei A, Ziarani GM, Tarlani A (2004) Novel method for the fast preconcentration and monitoring of a ppt level of lead and copper with a modified hexagonal mesoporous silica compound and inductively coupled plasma atomic emission spectrometry. Anal Sci 20:725–729
Vaisanen A, Suontamo R, Silvonen J, Rintala J (2002) Ultrasound-assisted extraction in the determination of arsenic, cadmium, copper, lead, and silver in contaminated soil samples by inductively coupled plasma atomic emission spectrometry. Anal Bioanal Chem 373:93–97
Hepp NM (2015) Determination of arsenic, chromium, lead, manganese, and mercury in certifiable color additives by inductively coupled plasma/mass spectrometry. J AOAC Int 98:160–164
Chen Y, Huang L, Wu W, Ruan Y, Wu Z, Xue Z, Fu F (2014) Speciation analysis of lead in marine animals by using capillary electrophoresis couple online with inductively coupled plasma mass spectrometry. Electrophoresis 35:1346–1352
Yilmaz V, Arslan Z, Rose L (2013) Determination of lead by hydride generation inductively coupled plasma mass spectrometry (HG-ICP-MS): on-line generation of plumbane using potassium hexacyanomanganate(III). Anal Chim Acta 761:18–26
Amarasiriwardena CJ, Jayawardene I, Lupoli N, Barnes RM, Hernandez-Avila M, Hu H, Ettinger AS (2013) Comparison of digestion procedures and methods for quantification of trace lead in breast milk by isotope dilution inductively coupled plasma mass spectrometry. Anal Methods 5:1676–1681
Vassileva E, Hoenig M (2011) Determination of the total and extractable mass fractions of cadmium and lead in mineral feed by using isotope dilution inductively coupled plasma mass spectrometry. Anal Chim Acta 701:37–44
Sun Y, Lu X, Su F, Wang L, Liu C, Duan X, Li Z (2015) Real-time fluorescence ligase chain reaction for sensitive detection of single nucleotide polymorphism based on fluorescence resonance energy transfer. Biosens Bioelectron 74:705–710
Demchenko AP (2013) Nanoparticles and nanocomposites for fluorescence sensing and imaging. Methods Appl Fluoresc 1:022001(28pp)
Miller JN (2005) Fluorescence energy transfer methods in bioanalysis. Analyst 130:265–270
Wang C, Cheng H, Sun Y, Xu Z, Lin H, Lin Q, Zhang C (2015) Nanoclusters prepared from a silver/gold alloy as a fluorescent probe for selective and sensitive determination of lead(II). Microchim Acta 182:695–701
Xu J, Li Y, Guo J, Shen F, Luo Y, Sun C (2014) Fluorescent detection of clenbuterol using fluorophore functionalized gold nanoparticles. Food Control 46:67–74
Cao X, Shen F, Zhang M, Guo J, Luo Y, Xu J, Li Y, Sun C (2014) Highly sensitive detection of melamine based on fluorescence resonance energy transfer between rhodamine B and gold nanoparticles. Dyes Pigments 111:99–107
Darbha GK, Ray A, Ray PC (2007) Gold nanoparticle-based miniaturized nanomaterial surface energy transfer probe for rapid and ultrasensitive detection of mercury in soil, water, and fish. ACS Nano 1:208–214
Farzampour L, Amjadi M (2014) Sensitive turn-on fluorescence assay of methimazole based on the fluorescence resonance energy transfer between acridine orange and silver nanoparticles. J Lumin 155:226–230
Zheng AF, Chen JL, Wu GN, Wei HCY, Kai XM, Wu GH, Chen Y (2009) Optimization of a sensitive method for the “switch-on” determination of mercury(II) in waters using rhodamine B capped gold nanoparticles as a fluorescence sensor. Microchim Acta 164:17–27
Yan YQ, Tang X, Wang YS, Li MH, Cao JX, Chen SH, Zhu YF, Wang XF, Huang YQ (2015) A sensitive and selective fluorescence assay for metallothioneins by exploiting the surface energy transfer between rhodamine 6G and gold nanoparticles. Microchim Acta 182:1353–1360
Zhou B, Wang YS, Yang HX, Xue JH, Wang JC, Liu SD, Liu H, Zhao H (2014) A sensitive resonance light scattering assay for uranyl ion based on the conformational change of a nuclease-resistant aptamer and gold nanoparticles acting as signal reporters. Microchim Acta 181:1353–1360
Guan Y, Zhou W, Yao X, Zhao M, Li Y (2006) Determination of nucleic acids based on the fluorescence quenching of hoechst 33258 at pH 4.5. Anal Chim Acta 570:21–28
Wang CI, Huang CC, Lin YW, Chen WT, Chang HT (2012) Catalytic gold nanoparticles for fluorescent detection of mercury(II) and lead(II) ions. Anal Chim Acta 745:124–130
Sitko R, Turek E, Zawisza B, Malicka E, Talik E, Heimann J, Gagor A, Feist B, Wrzalik R (2013) Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans 42:5682–5689
Acknowledgments
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (No. 21177052), the Science and Technology Program of Hunan Province in China (No. 2010SK3039) and the Construct Program of the Key Discipline (Public Health and Preventive Medicine) in Hunan Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Xiao-Feng Wang and Li-Ping Xiang are co-first authors.
Electronic supplementary material
ESM 1
(DOC 620 kb)
Rights and permissions
About this article
Cite this article
Wang, XF., Xiang, LP., Wang, YS. et al. A “turn-on” fluorescence assay for lead(II) based on the suppression of the surface energy transfer between acridine orange and gold nanoparticles. Microchim Acta 183, 1333–1339 (2016). https://doi.org/10.1007/s00604-015-1738-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1738-7