Abstract
The study of membrane proteins as prime drug targets has led to intensified efforts to characterize their structure and function. With regards to the structural analysis of membrane proteins, there have been considerable technological innovations in cryo-EM and X-ray crystallography, but advancements in the elucidation of membrane protein function, especially on a single-molecule level, have been struggling to bridge from basic science to high-throughput applications. There is a need for advanced biosensor platforms allowing membrane protein-mediated transport and potential suppressor libraries to be characterized. Membrane proteins facilitating the translocation of non-electrogenic substrates particularly suffer from a lack of such techniques to date. Here, we summarize recent developments in the field of membrane protein analysis, with a special focus on micro- and nanostructured platforms for purpose of high-throughput screening using fluorescent read-out systems. Additionally, their use as novel biosensor platforms to elucidate non-electrogenic substrate translocation is described. This overview contains 82 references.

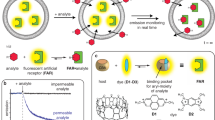
Micro- and nanostructured surfaces provide highly parallel sensor systems for the study of transmembrane processes. Deploying multiple fluorescence signals as readout, multiplexing can be achieved and even non-electrogenic substrate translocation detected in real-time, paving the way for high-content studies.

Similar content being viewed by others
References
Yıldırım MA, Goh K-I, Cusick ME, Barabási A-L, Vidal M (2007) Drug—target network. Nat Biotechnol 25(10):1119
Arinaminpathy Y, Khurana E, Engelman DM, Gerstein MB (2009) Computational analysis of membrane proteins: the largest class of drug targets. Drug Discov Today 14(23):1130–1135
Rawlins MD (2004) Cutting the cost of drug development? Nat Rev Drug Discov 3(4):360–364
Nathan C (2004) Antibiotics at the crossroads. Nature 431(7011):899–902
Morgan S, Grootendorst P, Lexchin J, Cunningham C, Greyson D (2011) The cost of drug development: a systematic review. Health Policy 100(1):4–17
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth F (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch EJP 391(2):85–100
Osaki T, Suzuki H, Le Pioufle B, Takeuchi S (2009) Multichannel simultaneous measurements of single-molecule translocation in α-hemolysin nanopore array. Anal Chem 81(24):9866–9870
Carrillo L, Cucu B, Bandmann V, Homann U, Hertel B, Hillmer S, Thiel G, Bertl A (2015) High‐resolution membrane capacitance measurements for studying endocytosis and exocytosis in yeast. Traffic. doi:10.1111/tra.12275
Fertig N, Blick RH, Behrends JC (2002) Whole cell patch clamp recording performed on a planar glass chip. Biophys J 82(6):3056–3062. doi:10.1016/S0006-3495(02)75646-4
Stoelzle S, Obergrussberger A, Brüggemann A, Haarmann C, George M, Kettenhofen R, Fertig N (2011) State-of-the-art automated patch clamp devices: heat activation, action potentials, and high throughput in ion channel screening. Front Pharmacol 2
Schroeder K, Neagle B, Trezise DJ, Worley J (2003) IonWorks (TM) HT: a new high-throughput electrophysiology measurement platform. J Biomol Screen 8(1):50–64. doi:10.1177/1087057102239667
Dunlop J, Bowlby M, Peri R, Vasilyev D, Arias R (2008) High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nat Rev Drug Discov 7(4):358–368
Uehlein N, Otto B, Eilingsfeld A, Itel F, Meier W, Kaldenhoff R (2012) Gas-tight triblock-copolymer membranes are converted to CO2 permeable by insertion of plant aquaporins. Sci Rep 2
Horn C, Steinem C (2005) Photocurrents generated by bacteriorhodopsin adsorbed on nano-black lipid membranes. Biophys J 89(2):1046–1054
Weiskopf D, Schmitt EK, Klühr MH, Dertinger SK, Steinem C (2007) Micro-BLMs on highly ordered porous silicon substrates: rupture process and lateral mobility. Langmuir 23(18):9134–9139
Kalsi S, Powl AM, Wallace BA, Morgan H, de Planque MR (2014) Shaped apertures in photoresist films enhance the lifetime and mechanical stability of suspended lipid bilayers. Biophys J 106(8):1650–1659
Funakoshi K, Suzuki H, Takeuchi S (2006) Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal Chem 78(24):8169–8174
Holden MA, Needham D, Bayley H (2007) Functional bionetworks from nanoliter water droplets. J Am Chem Soc 129(27):8650–8655
Bayley H, Cronin B, Heron A, Holden MA, Hwang WL, Syeda R, Thompson J, Wallace M (2008) Droplet interface bilayers. Mol Biosyst 4(12):1191–1208
Czekalska MA, Kaminski TS, Jakiela S, Sapra KT, Bayley H, Garstecki P (2015) A droplet microfluidic system for sequential generation of lipid bilayers and transmembrane electrical recordings. Lab Chip 15(2):541–548
Castell OK, Berridge J, Wallace MI (2012) Quantification of membrane protein inhibition by optical ion flux in a droplet interface bilayer array. Angew Chem Int Ed 51(13):3134–3138
Nisisako T, Portonovo SA, Schmidt JJ (2013) Microfluidic passive permeability assay using nanoliter droplet interface lipid bilayers. Analyst 138(22):6793–6800. doi:10.1039/C3AN01314F
Poulos JL, Jeon T-J, Damoiseaux R, Gillespie EJ, Bradley KA, Schmidt JJ (2009) Ion channel and toxin measurement using a high throughput lipid membrane platform. Biosensors Bioelectron 24(6):1806–1810. doi:10.1016/j.bios.2008.08.041
Jeon T-J, Malmstadt N, Schmidt JJ (2006) Hydrogel-encapsulated lipid membranes. J Am Chem Soc 128(1):42–43. doi:10.1021/ja056901v
X-f K, Cheley S, Rice-Ficht AC, Bayley H (2007) A storable encapsulated bilayer chip containing a single protein nanopore. J Am Chem Soc 129(15):4701–4705. doi:10.1021/ja068654g
Tamm LK, McConnell HM (1985) Supported phospholipid bilayers. Biophys J 47(1):105–113
Sackmann E (1996) Supported membranes: scientific and practical applications. Science 271(5245):43–48
Cremer PS, Boxer SG (1999) Formation and spreading of lipid bilayers on planar glass supports. J Phys Chem B 103(13):2554–2559
Castellana ET, Cremer PS (2006) Solid supported lipid bilayers: from biophysical studies to sensor design. Surf Sci Rep 61(10):429–444
Tanaka M, Sackmann E (2006) Supported membranes as biofunctional interfaces and smart biosensor platforms. Phys Status Solidi A Appl Res 203(14):3452–3462
Janshoff A, Steinem C (2006) Transport across artificial membranes—an analytical perspective. Anal Bioanal Chem 385(3):433–451
Reimhult E, Kumar K (2008) Membrane biosensor platforms using nano- and microporous supports. Trends Biotechnol 26(2):82–89. doi:10.1016/j.tibtech.2007.11.004
Suzuki H, Takeuchi S (2008) Microtechnologies for membrane protein studies. Anal Bioanal Chem 391(8):2695–2702
He L, Robertson JW, Li J, Kärcher I, Schiller SM, Knoll W, Naumann R (2005) Tethered bilayer lipid membranes based on monolayers of thiolipids mixed with a complementary dilution molecule. 1. Incorporation of channel peptides. Langmuir 21(25):11666–11672
Purrucker O, Hillebrandt H, Adlkofer K, Tanaka M (2001) Deposition of highly resistive lipid bilayer on silicon–silicon dioxide electrode and incorporation of gramicidin studied by ac impedance spectroscopy. Electrochim Acta 47(5):791–798
Rhoten MC, Burgess JD, Hawkridge FM (2002) The reaction of cytochrome c from different species with cytochrome c oxidase immobilized in an electrode supported lipid bilayer membrane. J Electroanal Chem 534(2):143–150
Bin X, Zawisza I, Goddard JD, Lipkowski J (2005) Electrochemical and PM-IRRAS studies of the effect of the static electric field on the structure of the DMPC bilayer supported at a Au (111) electrode surface. Langmuir 21(1):330–347
Devadoss A, Palencsár MS, Jiang D, Honkonen ML, Burgess JD (2005) Enzyme modification of platinum microelectrodes for detection of cholesterol in vesicle lipid bilayer membranes. Anal Chem 77(22):7393–7398
Bazzone A, Costa WS, Braner M, Călinescu O, Hatahet L, Fendler K (2013) Introduction to solid supported membrane based electrophysiology. J Vis Exp (75)
Mager T, Braner M, Kubsch B, Hatahet L, Alkoby D, Rimon A, Padan E, Fendler K (2013) Differential effects of mutations on the transport properties of the Na+/H+ antiporter NhaA from Escherichia coli. J Biol Chem 288(34):24666–24675
Wagner ML, Tamm LK (2000) Tethered polymer-supported planar lipid bilayers for reconstitution of integral membrane proteins: silane-polyethyleneglycol-lipid as a cushion and covalent linker. Biophys J 79(3):1400–1414
Naumann CA, Prucker O, Lehmann T, Rühe J, Knoll W, Frank CW (2002) The polymer-supported phospholipid bilayer: tethering as a new approach to substrate-membrane stabilization. Biomacromolecules 3(1):27–35
Naumann R, Schiller SM, Giess F, Grohe B, Hartman KB, Kärcher I, Köper I, Lübben J, Vasilev K, Knoll W (2003) Tethered lipid bilayers on ultraflat gold surfaces. Langmuir 19(13):5435–5443
Kuhner M, Tampe R, Sackmann E (1994) Lipid mono- and bilayer supported on polymer films: composite polymer-lipid films on solid substrates. Biophys J 67(1):217–226. doi:10.1016/S0006-3495(94)80472-2
Hillebrandt H, Wiegand G, Tanaka M, Sackmann E (1999) High electric resistance polymer/lipid composite films on indium-tin-oxide electrodes. Langmuir 15(24):8451–8459
Tanaka M, Sackmann E (2005) Polymer-supported membranes as models of the cell surface. Nature 437(7059):656–663
Purrucker O, Gönnenwein S, Förtig A, Jordan R, Rusp M, Bärmann M, Moroder L, Sackmann E, Tanaka M (2007) Polymer-tethered membranes as quantitative models for the study of integrin-mediated cell adhesion. Soft Matter 3(3):333–336
Stamou D, Duschl C, Delamarche E, Vogel H (2003) Self‐assembled microarrays of attoliter molecular vessels. Angew Chem Int Ed 115(45):5738–5741
Christensen SM, Stamou D (2007) Surface-based lipid vesicle reactor systems: fabrication and applications. Soft Matter 3(7):828–836
Christensen SM, Stamou DG (2010) Sensing-applications of surface-based single vesicle arrays. Sensors 10(12):11352–11368
Bolinger P-Y, Stamou D, Vogel H (2004) Integrated nanoreactor systems: triggering the release and mixing of compounds inside single vesicles. J Am Chem Soc 126(28):8594–8595
Christensen SM, Bolinger P-Y, Hatzakis NS, Mortensen MW, Stamou D (2012) Mixing subattolitre volumes in a quantitative and highly parallel manner with soft matter nanofluidics. Nat Nanotechnol 7(1):51–55
Christensen AL, Lohr C, Christensen SM, Stamou D (2013) Single vesicle biochips for ultra-miniaturized nanoscale fluidics and single molecule bioscience. Lab Chip 13(18):3613–3625
Dekker C (2007) Solid-state nanopores. Nat Nanotechnol 2(4):209–215
Howorka S, Siwy Z (2009) Nanopore analytics: sensing of single molecules. Chem Soc Rev 38(8):2360–2384
Basit H, Gaul V, Maher S, Forster RJ, Keyes TE (2015) Aqueous-filled polymer microcavity arrays: versatile & stable lipid bilayer platforms offering high lateral mobility to incorporated membrane proteins. Analyst 140(9):3012–3018
Simon A, Girard-Egrot A, Sauter F, Pudda C, D’Hahan NP, Blum L, Chatelain F, Fuchs A (2007) Formation and stability of a suspended biomimetic lipid bilayer on silicon submicrometer-sized pores. J Colloid Interface Sci 308(2):337–343
Han X, Studer A, Sehr H, Geissbühler I, Di Berardino M, Winkler FK, Tiefenauer LX (2007) Nanopore arrays for stable and functional free‐standing lipid bilayers. Adv Mater 19(24):4466–4470
Schmidt C, Mayer M, Vogel H (2000) A chip‐based biosensor for the functional analysis of single ion channels. Angew Chem Int Ed 112(17):3267–3270
Buchholz K, Tinazli A, Kleefen A, Dorfner D, Pedone D, Rant U, Tampé R, Abstreiter G, Tornow M (2008) Silicon-on-insulator based nanopore cavity arrays for lipid membrane investigation. Nanotechnology 19(44):445305
Kleefen A, Pedone D, Grunwald C, Wei R, Firnkes M, Abstreiter G, Rant U, Tampé R (2010) Multiplexed parallel single transport recordings on nanopore arrays. Nano Lett 10(12):5080–5087
Wu H-L, Chen P-Y, Chi C-L, Tsao H-K, Sheng Y-J (2013) Vesicle deposition on hydrophilic solid surfaces. Soft Matter 9(6):1908–1919
Kumar K, Isa L, Egner A, Schmidt R, Textor M, Reimhult E (2011) Formation of nanopore-spanning lipid bilayers through liposome fusion. Langmuir 27(17):10920–10928. doi:10.1021/la2019132
Urban M, Kleefen A, Mukherjee N, Seelheim P, Windschiegl B, Vor der Brüggen M, Koçer A, Tampé R (2014) Highly parallel transport recordings on a membrane-on-nanopore chip at single molecule resolution. Nano Lett 14(3):1674–1680
Kaufeld T, Steinem C, Schmidt CF (2015) Microporous device for local electric recordings on model lipid bilayers. J Phys D Appl Phys 48(2):025401
Kusters I, Van Oijen AM, Driessen AJ (2014) Membrane-on-a-chip: microstructured silicon/silicon-dioxide chips for high-throughput screening of membrane transport and viral membrane fusion. ACS Nano 8(4):3380–3392
Frese D, Steltenkamp S, Schmitz S, Steinem C (2013) In situ generation of electrochemical gradients across pore-spanning membranes. RSC Adv
Danelon C, Perez J-B, Santschi C, Brugger J, Vogel H (2006) Cell membranes suspended across nanoaperture arrays. Langmuir 22(1):22–25
Hennesthal C, Drexler J, Steinem C (2002) Membrane‐suspended nanocompartments based on ordered pores in alumina. Chemphyschem 3(10):885–889
Drexler J, Steinem C (2003) Pore-suspending lipid bilayers on porous alumina investigated by electrical impedance spectroscopy. J Phys Chem B 107(40):11245–11254
Römer W, Lam YH, Fischer D, Watts A, Fischer WB, Göring P, Wehrspohn RB, Gösele U, Steinem C (2004) Channel activity of a viral transmembrane peptide in micro-BLMs: Vpu1-32 from HIV-1. J Am Chem Soc 126(49):16267–16274
Römer W, Steinem C (2004) Impedance analysis and single-channel recordings on nano-black lipid membranes based on porous alumina. Biophys J 86(2):955–965
Schmitt EK, Vrouenraets M, Steinem C (2006) Channel activity of OmpF monitored in nano-BLMs. Biophys J 91(6):2163–2171
Steltenkamp S, Müller MM, Deserno M, Hennesthal C, Steinem C, Janshoff A (2006) Mechanical properties of pore-spanning lipid bilayers probed by atomic force microscopy. Biophys J 91(1):217–226
Hennesthal C, Steinem C (2000) Pore-spanning lipid bilayers visualized by scanning force microscopy. J Am Chem Soc 122(33):8085–8086
Stutzmann M, Garrido JA, Eickhoff M, Brandt MS (2006) Direct biofunctionalization of semiconductors: a survey. Phys Status Solidi A Appl Res 203(14):3424–3437
Korman CE, Megens M, Ajo-Franklin CM, Horsley DA (2013) Nanopore-spanning lipid bilayers on silicon nitride membranes that seal and selectively transport ions. Langmuir 29(14):4421–4425. doi:10.1021/la305064j
Lazzara TD, Carnarius C, Kocun M, Janshoff A, Steinem C (2011) Separating attoliter-sized compartments using fluid pore-spanning lipid bilayers. ACS Nano 5(9):6935–6944. doi:10.1021/nn201266e
Watanabe R, Soga N, Yamanaka T, Noji H (2014) High-throughput formation of lipid bilayer membrane arrays with an asymmetric lipid composition. Sci Rep 4
Sumitomo K, McAllister A, Tamba Y, Kashimura Y, Tanaka A, Shinozaki Y, Torimitsu K (2012) Ca2+ ion transport through channels formed by α-hemolysin analyzed using a microwell array on a Si substrate. Biosensors Bioelectron 31(1):445–450. doi:10.1016/j.bios.2011.11.010
Watanabe R, Soga N, Fujita D, Tabata KV, Yamauchi L, Kim SH, Asanuma D, Kamiya M, Urano Y, Suga H (2014) Arrayed lipid bilayer chambers allow single-molecule analysis of membrane transporter activity. Nat Commun 5
Kolesinska B, Podwysocka DJ, Rueping MA, Seebach D, Kamena F, Walde P, Sauer M, Windschiegl B, Meyer‐Ács M, Vor der Brüggen M (2013) Permeation through phospholipid bilayers, skin‐cell penetration, plasma stability, and cd spectra of α‐and β‐oligoproline derivatives. Chem Biodivers 10(1):1–38
Acknowledgments
The work was supported by the German Research Foundation Grant (CRC 807 – Transport and Communication across Biological Membranes; EXC 115 – Macromolecular Complexes), the German-Israeli Project Cooperation (DIP), and the Federal Ministry of Economics and Technology (ZIM R&D Project).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urban, M., Tampé, R. Membranes on nanopores for multiplexed single-transporter analyses. Microchim Acta 183, 965–971 (2016). https://doi.org/10.1007/s00604-015-1676-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1676-4