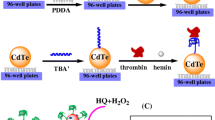
Abstract
We describe a near-infrared (NIR) fluorescent thrombin assay using a thrombin-binding aptamer (TBA) and Zn(II)-activated CuInS2 quantum dots (Q-dots). The fluorescence of Zn(II)-activated Q-dots is quenched by the TBA via photoinduced electron transfer, but if thrombin is added, it will bind to TBA to form G-quadruplexes and the Q-dots are released. As a result, the fluorescence intensity of the system is restored. This effect was exploited to design an assay for thrombin whose calibration plot, under optimum conditions, is linear in the 0.034 to 102 nmol L−1 concentration range, with a 12 pmol L−1 detection limit. The method is fairly simple, fast, and due to its picomolar detection limits holds great potential in the diagnosis of diseases associated with coagulation abnormalities and certain kinds of cancer.

We developed a simple near-infrared fluorescence assay using thrombin binding aptamer (TBA) and Zn(II)-activated CuInS2 quantum dots for the highly selective and sensitive detection of thrombin.






Similar content being viewed by others
References
Shuman MA (1986) Thrombin-cellular interactions. Ann NY Acad Sci 485:228–239
Xue YH, Zhang XF, Dong QZ, Sun JA, Dai C, Zhou HJ, Ren N, Jia HL, Ye QH, Qin LX (2010) Thrombin is a therapeutic target for metastatic osteopontin-positive hepatocellular carcinoma. Hepatology 52:2012–2022
Oroval M, Climent E, Coll C, Eritja R, Avino A, Marcos MD, Sancenon F, Martinez-Manez R, Amoros P (2013) An aptamer-gated silica mesoporous material for thrombin detection. Chem Commun 49:5480–5482
Ebert MPA, Lamer S, Meuer J, Malfertheiner P, Reymond M, Buschmann T, Rocken C, Seibert V (2005) Identification of the thrombin light chain A as the single best mass for differentiation of gastric cancer patients from individuals with dyspepsia by proteome analysis. J Proteome Res 4:586–590
Xu H, Mao X, Zeng QX, Wang SF, Kawde AN, Liu GD (2009) Aptamer-functionalized gold nanoparticles as probes in a dry-reagent strip biosensor for protein analysis. Anal Chem 81:669–675
Li LD, Zhao HT, Chen ZB, Mu XJ, Guo L (2010) Aptamer-based electrochemical approach to the detection of thrombin by modification of gold nanoparticles. Anal Bioanal Chem 398:563–570
Hu J, Zheng PC, Jiang JH, Shen GL, Yu RQ, Liu GK (2009) Electrostatic interaction based approach to thrombin detection by surface-enhanced raman spectroscopy. Anal Chem 81:87–93
Bo FL, Gao BX, Duan WF, Li HX, Liu HM, Bai QQ (2013) Assembly-disassembly driven “off-on” fluorescent perylene bisimide probes for detecting and tracking of proteins in living cells. RSC Adv 3:17007–17010
Raichlin S, Sharon E, Freeman R, Tzfati Y, Willner I (2011) Electron-transfer quenching of nucleic acid-functionalized CdSe/ZnS quantum dots by doxorubicin: a versatile system for the optical detection of DNA, aptamer-substrate complexes and telomerase activity. Biosens Bioelectron 26:4681–4689
Liao DL, Chen J, Li WY, Zhang QF, Wang FY, Li YX, Yu C (2013) Fluorescence turn-on detection of a protein using cytochrome c as a quencher. Chem Commun 49:9458–9460
Zhang CL, Xu J, Zhang SM, Ji XH, He ZK (2012) One-pot synthesized DNA-CdTe quantum dots applied in a biosensor for the detection of sequence-specific oligonucleotides. Chem Eur J 18:8296–8300
Iliuk AB, Hu LH, Tao WA (2011) Aptamer in bioanalytical applications. Anal Chem 83:4440–4452
Tang QJ, Su XD, Loh KP (2007) Surface plasmon resonance spectroscopy study of interfacial binding of thrombin to antithrombin DNA aptamers. J Colloid Interface Sci 315:99–106
Shan Y, Xu JJ, Chen HY (2011) Enhanced electrochemiluminescence quenching of CdS:Mn nanocrystals by CdTe QDs-doped silica nanoparticles for ultrasensitive detection of thrombin. Nanoscale 3:2916–2923
Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ (1992) Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 355:564–566
Smirnov I, Shafer RH (2000) Effect of loop sequence and size on DNA aptamer stability. Biochemistry 39:1462–1468
Zhang Y, Hong G, Zhang Y, Chen G, Li F, Dai H, Wang Q (2012) Ag2S quantum dot: a bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS Nano 6:3695–3702
Gao J, Chen K, Xie R, Xie J, Yan Y, Cheng Z, Peng X, Chen X (2010) In vivo tumor-targeted fluorescence imaging using near-infrared non-cadmium quantum dots. Bioconjug Chem 21:604–609
Park J, Dvoracek C, Lee KH, Galloway JF, Bhang HC, Pomper MG, Searson PC (2011) CuInSe/ZnS core/shell NIR quantum dots for biomedical imaging. Small 7:3148–3152
Au GHT, Shih WY, Tseng SJ, Shih WH (2012) Aqueous CdPbS quantum dots for near-infrared imaging. Nanotechnology 23:275601–275609
Xie RG, Rutherford M, Peng XG (2009) Formation of high-quality I-III-VI semiconductor nanocrystals by tuning relative reactivity of cationic precursors. J Am Chem Soc 131:5691–5697
Liu SY, Li YY, Su XG (2012) Determination of copper(II) and cadmium(II) based on ternary CuInS2 quantum dots. Anal Methods 4:1365–1370
Chen YF, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74:5132–5138
Niu SY, Qu LJ, Zhang Q, Lin JH (2012) Fluorescence detection of thrombin using autocatalytic strand displacement cycle reaction and a dual-aptamer DNA sandwich assay. Anal Biochem 421:362–367
Booth M, Brown AP, Evans SD, Critchley K (2012) Determining the concentration of CuInS2 quantum dots from the size-dependent molar extinction coefficient. Chem Mater 24:2064–2070
Yu WW, Qu LH, Guo WZ, Peng XG (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Xie R, Rutherford M, Peng X (2009) Formation of high-quality I− III− VI semiconductor nanocrystals by tuning relative reactivity of cationic precursors. J Am Chem Soc 131:5691–5697
Zhao Q, Lu X, Yuan C-G, Li X-F, Le XC (2009) Aptamer-linked assay for thrombin using gold nanoparticle amplification and inductively coupled plasma−mass spectrometry detection. Anal Chem 81:7484–7489
Chen Q, Tang W, Wang DZ, Wu XJ, Li N, Liu F (2010) Amplified QCM-D biosensor for protein based on aptamer-functionalized gold nanoparticles. Biosens Bioelectron 26:575–579
Li JWJ, Fang XH, Tan WH (2002) Molecular aptamer beacons for real-time protein recognition. Biochem Biophys Res Commun 292:31–40
Zhang Y, Tian J, Zhai J, Luo Y, Wang L, Li H, Sun X (2011) Fluorescence-enhanced potassium ions detection based on inherent quenching ability of deoxyguanosines and K+−induced conformational transition of G-rich ssDNA from duplex to G-quadruplex structures. J Fluoresc 21:1841–1846
Uhrovcik J (2014) Strategy for determination of LOD and LOQ values—some basic aspects. Talanta 119:178–180
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21075050, No. 21275063) and the science and technology development project of Jilin province, China (No. 20110334).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zihan Lin and Dong Pan contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2959 kb)
Rights and permissions
About this article
Cite this article
Lin, Z., Pan, D., Hu, T. et al. A near-infrared fluorescent bioassay for thrombin using aptamer-modified CuInS2 quantum dots. Microchim Acta 182, 1933–1939 (2015). https://doi.org/10.1007/s00604-015-1526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1526-4