Abstract
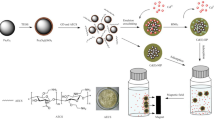
A surface-imprinting technique combined with a sacrificial support process was established to synthesize a novel Ce(III)-imprinted polymer (CIP) in which attapulgite acts as the sacrificial support material. The CIP was compared with attapulgite, non-imprinted polymer (NIP), and with a Ce(III)-imprinted polymer where attapulgite acts as the support material (AIP). Fourier transmission infrared spectrometry, scanning electron microscopy, transmission electron microscopy, simultaneous thermogravimetry, nitrogen sorption, and laser particle sizing were employed, and an imprinting mechanism is suggested. Batch experiments were performed to evaluate adsorption kinetics, selective recognition, adsorption isotherms, desorption and regeneration performances of the CIP. The CIP offers fast adsorption kinetics for Ce3+, and the maximum adsorption capacity is 130 mg g−1, which is larger than that of AIP and attapulgite. The absorption abilities of Ce3+ from aqueous solutions followed the order CIP>AIP>attapulgite>NIP. CIP could be reused four times with only about 16% and 18% loss of adsorption capacity in pure Ce3+ solution and potentially interfering ion solution, respectively. The method was applied to the separation and determination of trace Ce3+ in river sediments. The relative standard deviation of the method is 2.6% (n = 6.0), and the detection limit (3σ) is 57 ng L−1.






Similar content being viewed by others
References
Majdan M, Pikus S, Gladysz-Plaska A, Fuks L, Ziba E (2002) Adsorption of light lanthanides on the zeolite A surface. Colloids Surf A 209:27–35
Dhara S, Sarkar S, Basu S, Chattopadhyay P (2009) Separation of the 90Sr-90Y pair with cerium(IV) iodotungstate cation exchanger. Appl Radiat Isot 67:530–534
Jain VK, Handa A, Sait SS, Shrivastav P, Agrawal YK (2001) Pre-concentration, separation and trace determination of lanthanum(III), cerium(III), thorium(IV) and uranium(VI) on polymer supported o-vanillinsemicarbazone. Anal Chim Acta 429:237–246
Vasudevan S, Sozhan G, Mohan S, Pushpavanam S (2005) An electrochemical process for the separation of cerium from rare earths. Hydrometallurgy 76:115–121
Huang JH, Liu YF, Jin QZ, Wang XG, Yang J (2007) Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonication-surfactant-modified attapulgite clay. J Hazard Mater 143:541–548
Li Q, Su HJ, Tan TW (2008) Synthesis of ion-imprinted chitosan-TiO2 adsorbent and its multi-functional performances. Biochem Eng J 38:212–218
Tsukagoshi K, Yu KY, Maeda M, Takagi M (1993) Metal ion-selective adsorbent prepared by surface-imprinting polymerization. Bull Chem Soc Jpn 66:114–120
Ren YM, Zhang ML, Zhao D (2008) Synthesis and properties of magnetic Cu(II) ion imprinted composite adsorbent for selective removal of copper. Desalination 228:135–149
Wu GH, Wang ZQ, Wang J, He CY (2007) Hierarchically imprinted organic–inorganic hybrid sorbent for selective separation of mercury ion from aqueous solution. Anal Chim Acta 582:304–310
Chang XJ, Jiang N, Zheng H, He Q, Hu Z, Zhai YH, Cui YM (2007) Solid-phase extraction of iron(III) with an ion-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique. Talanta 71:38–43
Birlik E, Ersöz A, Açıkkalp E, Denizli A, Say R (2007) Preconcentration of phosphate ion onto ion-imprinted polymer. J Hazard Mater 140:110–136
Candan N, Tüzmen N, Andac M, Andac CA, Say R, Denizli A (2009) Cadmium removal out of human plasma using ion-imprinted beads in a magnetic column. Mater Sci Eng C 29:144–152
Wang ZQ, Wang M, Wu GH (2010) Ion imprinted sol-gel nanotubes membrane for selective separation of copper ion from aqueous solution. Microchim Acta 169:195–200
Zhao JC, Han B, Zhang YF, Wang DD (2007) Synthesis of Zn(II) ion-imprinted solid-phase extraction material and its analytical application. Anal Chim Acta 603:87–92
Shirvani-Arani S, Ahmadi SJ, Bahrami-Samani A, Ghannadi-Maragheh M (2008) Synthesis of nano-pore samarium (III)-imprinted polymer for preconcentrative separation of samarium ions from other lanthanide ions via solid phase extraction. Anal Chim Acta 623:82–88
He Q, Chang XJ, Wu Q, Huang XP, Hu Z, Zhai YZ (2007) Synthesis and applications of surface-grafted Th(IV)-imprinted polymers for selective solid-phase extraction of thorium(IV). Anal Chim Acta 605:192–197
Yilmaz E, Ramström O, Möller P, Sanchez D, Mosbach K (2002) A facile method for preparing molecularly imprinted polymer spheres using spherical silica templates. J Mater Chem 12:1577–1581
He CY, Long YY, Pan JL, Li K, Liu F (2008) Molecularly imprinted silica prepared with immiscible ionic liquid as solvent and porogen for selective recognition of testosterone. Talanta 74:1126–1131
Li F, Jiang HQ, Zhang SS (2007) An ion-imprinted silica-supported organic–inorganic hybrid sorbent prepared by a surface imprinting technique combined with a polysaccharide incorporated sol–gel process for selective separation of cadmium(II) from aqueous solution. Talanta 71:1487–1493
Li F, Li XM, Zhang SS (2006) One-pot preparation of silica-supported hybrid immobilized metal affinity adsorbent with macroporous surface based on surface imprinting coating technique combined with polysaccharide incorporated sol–gel process. J Chromatogr A 1129:223–230
Benhammou A, Yaacoubi L, Nibou B, Tanouti B (2005) Adsorption of metal ions onto Moroccan stevensite: kinetic and isotherm studies. J Colloid Interface Sci 282:320–326
Huang JH, Liu YF, Jin QZ, Wang XG, Yang J (2007) Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonication-surfactant-modified attapulgite clay. J Hazard Mater 143:541–548
Shen W, Chen SY, Shi SK, Xiang XL, Hu WL, Wang HP (2009) Adsorption of Cu(II) and Pb(II) onto diethylenetriamine-bacterial cellulose. Carbohydr Polym 75:110–114
Vigneau O, Pinel C, Lemaire M (2002) Solid-liquid separation of lanthanide/lanthanide and lanthanide/actinide using ionic imprinted polymer based on a DTPA derivative. Chem Lett 2:202–204
Tuzen M, Citaka D, Soylak M (2008) 5-Chloro-2-hydroxyaniline–copper(II) coprecipitation system for preconcentration and separation of lead(II) and chromium(III) at trace levels. J Hazard Mater 158:137–141
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.20877036).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Table S-1
Working conditions of ICP-AES. (DOC 31 kb)
Table S-2
The BET surface area and pore volume for polymers. (DOC 29 kb)
Table S-3
Langmuir adsorption isotherm constants for attapulgite, AIP and CIP. (DOC 30 kb)
Table S-4
Separation factor (R L) for adsorption Ce3+. (DOC 29 kb)
Table S-5
Effects of type, concentration and volume of desorption agent on the desorption Ce3+. (DOC 38 kb)
Table S-6
Effect of potentially interfering ions on the recovery of Ce3+. (DOC 32 kb)
Fig. S-1
Competitive adsorption for metal ions onto the CIP and NIP. (DOC 57 kb)
Fig. S-2
The adsorption capacity of CIP after four cycles regeneration. (DOC 61 kb)
Rights and permissions
About this article
Cite this article
Pan, J., Zou, X., Li, C. et al. Synthesis and applications of Ce(III)-imprinted polymer based on attapulgite as the sacrificial support material for selective separation of cerium(III) ions. Microchim Acta 171, 151–160 (2010). https://doi.org/10.1007/s00604-010-0416-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0416-z