Abstract
Purpose
Postoperative stress produces an inflammatory response. Recent studies have shown that narcotic analgesics suppress the immune system. Nutritional management during perioperative care has also been reported to affect inflammation. We therefore examined whether remifentanil or glucose administration could ameliorate postsurgical inflammatory responses using a rat model of surgical stress.
Methods
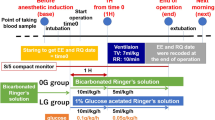
We divided male Wistar rats randomly into five groups: (1) control, (2) sevoflurane+lactated Ringer’s solution, (3) sevoflurane+lactated Ringer’s solution with 1% glucose, (4) sevoflurane+remifentanil+lactated Ringer’s solution, and (5) sevoflurane+remifentanil+ lactated Ringer’s solution with 1% glucose. In all groups, serum samples were obtained at various time points after surgery, and secreted cytokine concentrations were determined. In addition, we assessed the activation of protein kinase B (Akt) and forkhead/winged helix box class O (FOXO3), which play a role in gluconeogenesis/stress responses.
Results
Surgical stress increased the serum concentrations of tumor necrosis factor-α and interleukin-6. Groups receiving remifentanil with anesthesia showed an attenuated inflammatory response. The inflammatory response was also reduced by administering 1% glucose. Furthermore, 1% glucose induced Akt and FOXO3 phosphorylation in the quadriceps femoris muscle 12 h after surgery.
Conclusions
Anesthesia based on remifentanil and perioperative administration of lactated Ringer’s solution containing 1% glucose may be able to control inflammatory responses caused by surgical stress.
Similar content being viewed by others
References
Patrick DA, Moore FA, Moore EE, Biffi WL, Sauaia A, Barnett CC. The inflammatory profile of interleukin-6, interleukin-8, and soluble intercellular adhesion molecule-1 in postinjury multiple organ failure. Am J Surg 1996;172:425–431.
Wong GT, Li R, Jiang LL, Irwin MG. Remifentanil post-conditioning attenuates cardiac ischemia-reperfusion injury via kappa or delta opioid receptor activation. Acta Anaesthesiol Scand 2010;54:510–518.
Kim HS, Cho JE, Hong SW, Kim SO, Shim JK, Kwak YL. Remifentanil protects myocardium through activation of anti-apoptotic pathways of survival in ischemia-reperfused rat heart. Physiol Res 2010;59:347–356.
Bencsics A, Elenkov IJ, Vizi ES. Effect of morphine on lipopolysaccharide-induced tumor necrosis factor-production in vivo: involvement of the sympathetic nervous system. J Neuroimmunol 1997;73:1–6.
Murphy GS, Szokol JW, Marymont JH, Avram MJ, Vender JS. The effects of morphine and fentanyl on the inflammatory response to cardiopulmonary bypass in patients undergoing elective coronary artery bypass graft surgery. Anesth Analg 2007;104:1334–1342.
Crossland H, Constantin-Teodosiu D, Gardiner SM, Constantin D, Greenhaff PL. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J Physiol 2008;586:5589–5600.
Bezzi M, Hasmim M, Bieler G, Dormond O, Ruegg C. Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death: evidence for the suppression of sustained activation of focal adhesion kinase and protein kinase B/Akt. J Biol Chem 2003;278:43603–43614.
Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 2004;21:203–213.
Tremblay ML, Giguère V. Phosphatases at the heart of FoxO metabolic control. Cell Metab 2008;7:101–103.
Baigrie RJ, Lamont P, Dallman MJ, Kwiatkowski D, Morris PJ. The cytokine response to major surgery. Br J Surg 1992;79:757–760.
Ohzato H, Yoshizaki K, Nishimoto N, Ogata A, Tagoh H, Monden M, et al. Interleukin-6 is a new indicator of inflammatory status: detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery 1992;111:201–209.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367.
Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem 2000;275:17728–17739.
Hagiwara S, Iwasaka H, Hasegawa A, Koga H, Noguchi T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model. Crit Care Med 2008;36:2407–2413.
Tanaka T, Nabatame H, Tanifuji Y. Insulin secretion and glucose utilization are impaired under general anesthesia with sevoflurane as well as isoflurane in a concentration-independent manner. J Anesth 2005;19:277–281.
Saho S, Kadota Y, Sameshima T, Miyao J, Tsurumaru T, Yoshimura N. The effects of sevoflurane anesthesia on insulin secretion and glucose metabolism in pigs. Anesth Analg 1997;84:1359–1365.
Bonnet MP, Beloeil H, Benhamou D, Mazoit JX, Asehnoune K. The mu opioid receptor mediates morphine-induced tumor necrosis factor and interleukin-6 inhibition in toll-like receptor 2-stimulated monocytes. Anesth Analg 2008;106:1142–1149.
Farese RV. Insulin-sensitive phospholipid signaling systems and glucose transport. Update II. Exp Biol Med (Maywood) 2001;226:283–295.
Farese RV, Sajan MP, Standaert ML. Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): actions and defects in obesity and type II diabetes. Exp Biol Med 2005;230:593–605.
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 1998;279:710–714.
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer 2007;7:847–859.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hasegawa, A., Iwasaka, H., Hagiwara, S. et al. Remifentanil and glucose suppress inflammation in a rat model of surgical stress. Surg Today 41, 1617–1621 (2011). https://doi.org/10.1007/s00595-010-4457-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-010-4457-z