Abstract
Aims
To assess the metabolic health of obese and non-obese women at high GDM risk 5 years postpartum.
Methods
This is a secondary analysis of the 5-year follow-up of the RADIEL GDM prevention study including 333 women at high GDM risk (BMI ≥ 30 kg/m2 and/or previous GDM). Five years postpartum metabolic health was assessed including anthropometric measurements, oral glucose tolerance test, lipid metabolism, and body composition as well as medical history questionnaires. For the analysis, we divided the women into four groups based on parity, BMI, and previous history of GDM.
Results
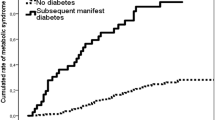
Five years postpartum impaired glucose regulation (IFG, IGT, or diabetes) was diagnosed in 15% of the women; 3.6% had type 2 diabetes. The highest prevalence was observed among obese women with a history of GDM (26%), and the lowest prevalence (8%) among primiparous obese women (p = 0.021). At follow-up 25–39% of the obese women fulfilled the diagnostic criteria for the metabolic syndrome, in the non-obese group 11% (p < 0.001). This was associated with body fat percentage. The non-obese group, however, faced metabolic disturbances (IFG, IGT, diabetes, or metabolic syndrome) at a significantly lower BMI (p < 0.001). Among women who were non-obese before pregnancy, 5 years postpartum, the obesity prevalence based on BMI was 14% and based on body fat percentage 58%.
Conclusions
The prevalence of impaired glucose regulation and metabolic syndrome is high 5 years postpartum among women at high risk of GDM. There are high-risk women also among the non-obese, who develop metabolic derangements already at a lower BMI.
Clinical trial registration
ClinicalTrials.gov, www.clinicaltrials.com, NCT01698385.




Similar content being viewed by others
References
National Institute for Health and Welfare (2016) Official Statistics of Finland; Health; 2014
Lamain-de Ruiter M, Kwee A, Naaktgeboren CA et al (2016) External validation of prognostic models to predict risk of gestational diabetes mellitus in one Dutch cohort: prospective multicentre cohort study. BMJ 354:4338. https://doi.org/10.1136/bmj.i4338
Tuomi T, Santoro N, Caprio S et al (2014) The many faces of diabetes: a disease with increasing heterogeneity. Lancet 383(9922):1084–1094. https://doi.org/10.1016/S0140-6736(13)62219-9
Freinkel N, Metzger BE, Phelps RL et al (1985) Gestational diabetes mellitus. Heterogeneity of maternal age, weight, insulin secretion, HLA antigens, and islet cell antibodies and the impact of maternal metabolism on pancreatic B-cell and somatic development in the offspring. Diabetes 34(Suppl 2):1–7
Bo S, Menato G, Pinach S et al (2003) Clinical characteristics and outcome of pregnancy in women with gestational hyperglycaemia with and without antibodies to beta-cell antigens. Diabet Med 20(1):64–68
Powe CE, Allard C, Battista M-C et al (2016) Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 39(6):1052–1055. https://doi.org/10.2337/dc15-2672
Huvinen E, Grotenfelt NE, Eriksson JG et al (2016) Heterogeneity of maternal characteristics and impact on gestational diabetes (GDM) risk—implications for universal GDM screening? Ann Med 48(1–2):52–58. https://doi.org/10.3109/07853890.2015.1131328
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373(9677):1773–1779. https://doi.org/10.1016/S0140-6736(09)60731-5
Puhkala J, Kinnunen TI, Vasankari T, Kukkonen-Harjula K, Raitanen J, Luoto R (2013) Prevalence of metabolic syndrome one year after delivery in Finnish women at increased risk for gestational diabetes mellitus during pregnancy. J Pregnancy 2013:139049
Hakkarainen H, Huopio H, Cederberg H, Paakkonen M, Voutilainen R, Heinonen S (2016) The risk of metabolic syndrome in women with previous GDM in a long-term follow-up. Gynecol Endocrinol. https://doi.org/10.1080/09513590.2016.1198764
Lauenborg J, Mathiesen E, Hansen T et al (2005) The prevalence of the metabolic syndrome in a danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab 90(7):4004–4010. https://doi.org/10.1210/jc.2004-1713
McKenzie-Sampson S, Paradis G, Healy-Profitos J, St-Pierre F, Auger N (2018) Gestational diabetes and risk of cardiovascular disease up to 25 years after pregnancy: a retrospective cohort study. Acta Diabetol. https://doi.org/10.1007/s00592-017-1099-2
Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA (1991) Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 165(6 Pt 1):1667–1672
Damm P, Kuhl C, Hornnes P, Molsted-Pedersen L (1995) A longitudinal study of plasma insulin and glucagon in women with previous gestational diabetes. Diabetes Care 18(5):654–665
Rono K, Stach-Lempinen B, Klemetti MM et al (2014) Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy Childbirth 14:70. https://doi.org/10.1186/1471-2393-14-70
Koivusalo SB, Rono K, Klemetti MM et al (2016) gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish Gestational Diabetes Prevention Study (RADIEL): a randomized controlled trial. Diabetes Care 39(1):24–30. https://doi.org/10.2337/dc15-0511
Alberti KGMM, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644
Malavolti M, Mussi C, Poli M et al (2003) Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann Hum Biol 30(4):380–391. https://doi.org/10.1080/0301446031000095211
USA: The American council on exercise (2016) What are the guidelines for percentage of body fat loss? http://www.acefitness.org/acefit/healthy-living-article/60/112/what-are-the-guidelines-for-percentage-of-body-fat. Accessed 22 Aug 2016
Noctor E, Crowe C, Carmody LA et al (2016) Abnormal glucose tolerance post-gestational diabetes mellitus as defined by the International Association of Diabetes and Pregnancy Study Groups criteria. Eur J Endocrinol 175(4):287–297. https://doi.org/10.1530/EJE-15-1260
Ekelund M, Shaat N, Almgren P, Groop L, Berntorp K (2010) Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia 53(3):452–457. https://doi.org/10.1007/s00125-009-1621-3
Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S (2016) Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia 59(7):1403–1411. https://doi.org/10.1007/s00125-016-3927-2
Kohler M, Ziegler AG, Beyerlein A (2016) Development of a simple tool to predict the risk of postpartum diabetes in women with gestational diabetes mellitus. Acta Diabetol 53(3):433–437. https://doi.org/10.1007/s00592-015-0814-0
Hakkarainen H, Huopio H, Cederberg H, Paakkonen M, Voutilainen R, Heinonen S (2015) Post-challenge glycemia during pregnancy as a marker of future risk of type 2 diabetes: a prospective cohort study. Gynecol Endocrinol 31(7):573–577. https://doi.org/10.3109/09513590.2015.1032926
Colditz GA, Willett WC, Rotnitzky A, Manson JE (1995) Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 122(7):481–486
Cho NH, Ahn CH, Moon JH, Kwak SH, Choi SH, Lim S, Park KS, Metzger BE, Jang HC (2016) Metabolic syndrome independently predicts future diabetes in women with a history of gestational diabetes mellitus. Medicine 95(35):e4582. https://doi.org/10.1097/MD.0000000000004582
Gobl CS, Bozkurt L, Prikoszovich T, Winzer C, Pacini G, Kautzky-Willer A (2011) Early possible risk factors for overt diabetes after gestational diabetes mellitus. Obstet Gynecol 118(1):71–78. https://doi.org/10.1097/AOG.0b013e318220e18f
Xu T, Liu J, Liu J, Zhu G, Han S (2017) Relation between metabolic syndrome and body compositions among Chinese adolescents and adults from a large-scale population survey. BMC Public Health 17(1):337. https://doi.org/10.1186/s12889-017-4238-3
Shah RV, Murthy VL, Abbasi SA et al (2014) Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA study. Jacc Cardiovasc Imaging 7(12):1221–1235. https://doi.org/10.1016/j.jcmg.2014.07.017
Franco LP, Morais CC, Cominetti C (2016) Normal-weight obesity syndrome: diagnosis, prevalence, and clinical implications. Nutr Rev 74(9):558–570. https://doi.org/10.1093/nutrit/nuw019
Romero-Corral A, Somers VK, Sierra-Johnson J et al (2010) Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 31(6):737–746. https://doi.org/10.1093/eurheartj/ehp487
Marques-Vidal P, Pecoud A, Hayoz D et al (2010) Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis 20(9):669–675. https://doi.org/10.1016/j.numecd.2009.06.001
De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L (2007) Normal-weight obese syndrome: early inflammation? Am J Clin Nutr 85(1):40–45
Huopio H, Hakkarainen H, Paakkonen M et al (2014) Long-term changes in glucose metabolism after gestational diabetes: a double cohort study. BMC Pregnancy Childbirth 14:296. https://doi.org/10.1186/1471-2393-14-296
Barker DJ (1990) The fetal and infant origins of adult disease. BMJ 301(6761):1111
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73. https://doi.org/10.1056/NEJMra0708473
Eriksson JG, Forsen T, Tuomilehto J, Jaddoe VWV, Osmond C, Barker DJP (2002) Effects of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia 45(3):342–348. https://doi.org/10.1007/s00125-001-0757-6
Sarr O, Yang K, Regnault TRH (2012) In utero programming of later adiposity: the role of fetal growth restriction. J Pregnancy 2012:134758. https://doi.org/10.1155/2012/134758
Entringer S, Buss C, Swanson JM et al (2012) Fetal programming of body composition, obesity, and metabolic function: the role of intrauterine stress and stress biology. J Nutr Metab 2012:632548. https://doi.org/10.1155/2012/632548
Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R (2013) Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med 30(12):1449–1456. https://doi.org/10.1111/dme.12286
Catalano PM, McIntyre HD, Cruickshank JK et al (2012) The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 35(4):780–786. https://doi.org/10.2337/dc11-1790
Benhalima K, Mathieu C, Van Assche A et al (2016) Survey by the European Board and College of Obstetrics and Gynaecology on screening for gestational diabetes in Europe. Eur J Obstet Gynecol Reprod Biol 201:197–202. https://doi.org/10.1016/j.ejogrb.2016.04.003
Farrar D, Simmonds M, Bryant M et al (2017) Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: a systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS ONE [Electronic Resource] 12(4):e0175288. https://doi.org/10.1371/journal.pone.0175288
Goueslard K, Cottenet J, Mariet AS, Sagot P, Petit JM, Quantin C (2017) Early screening for type 2 diabetes following gestational diabetes mellitus in France: hardly any impact of the 2010 guidelines. Acta Diabetol 54(7):645–651. https://doi.org/10.1007/s00592-017-0986-x
Ferrara A, Peng T, Kim C (2009) Trends in postpartum diabetes screening and subsequent diabetes and impaired fasting glucose among women with histories of gestational diabetes mellitus: a report from the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 32(2):269–274. https://doi.org/10.2337/dc08-1184
Acknowledgements
The study was funded by Ahokas Foundation, the Finnish Foundation for Cardiovascular Disease, Academy of Finland, Special state subsidy for health science research of Helsinki University Hospital (HUH), Samfundet Folkhälsan, Finska Läkaresällskapet, Juho Vainio Foundation, Viipuri Tuberculosis Foundation, The Finnish Diabetes Research Foundation, State Provincial Office of Southern Finland, Health Promotion Grant (Ministry of Social Affairs and Health) EU H2020-PHC-2014-DynaHealth Grant No. 633595, and The Social Insurance Institution of Finland. The funders have not had any role in designing or conducting the study; nor in collection, management, analysis, or interpretation of the data; nor in preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
EH participated in the design and implementation of the study, literature search, data interpretation, and the drafting and editing of the article. JGE is the principal investigator of the study, participated in the implementation of the study, analysis of the results, and advised on drafting and editing the article. SBK initiated, participated in the design of the study, coordinated the study, and helped in the editing of the article. NG participated in the design of the study and helped with the editing of the article. AT participated in the design of the study and helped with the editing of the article. BS-L participated in the design of the study, coordinated the study in Lappeenranta, and helped with editing the article. KR participated in the design of the study and helped in the statistical analysis and drafting and editing of the article. All authors have read and approved the final version of the manuscript. EH is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committees of HUH and SKCH and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Managed By Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huvinen, E., Eriksson, J.G., Koivusalo, S.B. et al. Heterogeneity of gestational diabetes (GDM) and long-term risk of diabetes and metabolic syndrome: findings from the RADIEL study follow-up. Acta Diabetol 55, 493–501 (2018). https://doi.org/10.1007/s00592-018-1118-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1118-y