Abstract
Purpose
There has been a growing interest in continuous local anaesthetic wound infiltration as a non-opioid technique for postoperative pain relief. The impact of this modality on baseline analgesia after spinal fusion surgery has however been inconclusive. We tested whether continuous wound infiltration with ropivacaine can enhance postoperative analgesia compared to a baseline intravenous multimodal analgesia protocol after spinal fusion surgery.
Methods
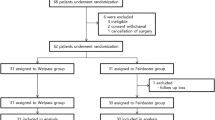
In this randomized, double-blinded, placebo-controlled study, a multiholed 19-gauge catheter was placed at the end of the surgical procedure through the wound to permit the continuous administration (8 ml/h) of ropivacaine 0.2 % (ropivacaine group; n = 19 patients) or saline (control group; n = 20 patients) during the first 48 postoperative hours (H48). Both groups received intraoperative low-dose ketamine, a combination of acetaminophen, non-steroidal anti-inflammatory drug, and nefopam over the same postoperative period, and morphine delivered by a patient-controlled analgesia (PCA) device.
Results
Morphine consumption was comparable between the two groups both at H48, 38 mg (26:52) (median, 25th:75th percentile) (control group) versus 43 mg (19:74) (ropivacaine group), and at H24, 18 mg (16:22) versus 22 mg (9:35) respectively. Pain scores at rest and during mobilization, quality of postoperative sleep, and morphine-related side effects were comparable between the two groups at H24 and H48.
Conclusion
Our findings indicate that no additional analgesia was provided with continuous wound infiltration of ropivacaine compared to a baseline intravenous multimodal analgesia protocol after spinal fusion surgery.
Trial registration
Clinicaltrials.gov #NCT01743794.



Similar content being viewed by others
References
Mathiesen O, Dahl B, Thomsen BA, Kitter B, Sonne N, Dahl JB, Kehlet H (2013) A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J 22(9):2089–2096. doi:10.1007/s00586-013-2826-1
Bajwa SJ, Haldar R (2015) Pain management following spinal surgeries: an appraisal of the available options. J Craniovertebr Junction Spine 6(3):105–110. doi:10.4103/0974-8237.161589
Aubrun F, Langeron O, Heitz D, Coriat P, Riou B (2000) Randomised, placebo-controlled study of the postoperative analgesic effects of ketoprofen after spinal fusion surgery. Acta Anaesthesiol Scand 44(8):934–939
Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, Beach ML (2010) Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology 113(3):639–646. doi:10.1097/ALN.0b013e3181e90914
Ersayli DT, Gurbet A, Bekar A, Uckunkaya N, Bilgin H (2006) Effects of perioperatively administered bupivacaine and bupivacaine-methylprednisolone on pain after lumbar discectomy. Spine 31(19):2221–2226. doi:10.1097/01.brs.0000232801.19965.a0
Jirarattanaphochai K, Jung S, Thienthong S, Krisanaprakornkit W, Sumananont C (2007) Peridural methylprednisolone and wound infiltration with bupivacaine for postoperative pain control after posterior lumbar spine surgery: a randomized double-blinded placebo-controlled trial. Spine 32(6):609–616. doi:10.1097/01.brs.0000257541.91728.a1
Garcia RM, Cassinelli EH, Messerschmitt PJ, Furey CG, Bohlman HH (2013) A multimodal approach for postoperative pain management after lumbar decompression surgery: a prospective, randomized study. J Spinal Disord Tech 26(6):291–297. doi:10.1097/BSD.0b013e318246b0a6
Kim SI, Ha KY, Oh IS (2015) Preemptive multimodal analgesia for postoperative pain management after lumbar fusion surgery: a randomized controlled trial. Eur Spine J. doi:10.1007/s00586-015-4216-3
Barron DJ, Tolan MJ, Lea RE (1999) A randomized controlled trial of continuous extra-pleural analgesia post-thoracotomy: efficacy and choice of local anaesthetic. Eur J Anaesthesiol 16(4):236–245
Dowling R, Thielmeier K, Ghaly A, Barber D, Boice T, Dine A (2003) Improved pain control after cardiac surgery: results of a randomized, double-blind, clinical trial. J Thorac Cardiovasc Surg 126(5):1271–1278. doi:10.1016/S0022
Wheatley GH 3rd, Rosenbaum DH, Paul MC, Dine AP, Wait MA, Meyer DM, Jessen ME, Ring WS, DiMaio JM (2005) Improved pain management outcomes with continuous infusion of a local anesthetic after thoracotomy. J Thorac Cardiovasc Surg 130(2):464–468. (pii S0022522305002060)
Beaussier M, El’Ayoubi H, Schiffer E, Rollin M, Parc Y, Mazoit JX, Azizi L, Gervaz P, Rohr S, Biermann C, Lienhart A, Eledjam JJ (2007) Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: a randomized, double-blind, placebo-controlled study. Anesthesiology 107(3):461–468. doi:10.1097/01.anes.0000278903.91986.19
Zohar E, Fredman B, Phillipov A, Jedeikin R, Shapiro A (2001) The analgesic efficacy of patient-controlled bupivacaine wound instillation after total abdominal hysterectomy with bilateral salpingo-oophorectomy. Anesth Analg 93(2):482–487
Bianconi M, Ferraro L, Ricci R, Zanoli G, Antonelli T, Giulia B, Guberti A, Massari L (2004) The pharmacokinetics and efficacy of ropivacaine continuous wound instillation after spine fusion surgery. Anesth Analg 98(1):166–172
Xu B, Ren L, Tu W, Wu Z, Ai F, Zhou D, Chen B, Zhang X (2015) Continuous wound infusion of ropivacaine for the control of pain after thoracolumbar spinal surgery: a randomized clinical trial. Eur Spine J. doi:10.1007/s00586-015-3979-x
Apfelbaum JL, Gan TJ, Zhao S, Hanna DB, Chen C (2004) Reliability and validity of the perioperative opioid-related symptom distress scale. Anesth Analg 99(3):699–709. doi:10.1213/01.ANE.0000133143.60584.38
Cheong WK, Seow-Choen F, Eu KW, Tang CL, Heah SM (2001) Randomized clinical trial of local bupivacaine perfusion versus parenteral morphine infusion for pain relief after laparotomy. Br J Surg 88(3):357–359. doi:10.1046/j.1365-2168.2001.01717.x
Bianconi M, Ferraro L, Traina GC, Zanoli G, Antonelli T, Guberti A, Ricci R, Massari L (2003) Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth 91(6):830–835
Liu SS, Richman JM, Thirlby RC, Wu CL (2006) Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg 203(6):914–932. (pii S1072-7515(06)01236-1)
Fredman B, Zohar E, Tarabykin A, Shapiro A, Mayo A, Klein E, Jedeikin R (2001) Bupivacaine wound instillation via an electronic patient-controlled analgesia device and a double-catheter system does not decrease postoperative pain or opioid requirements after major abdominal surgery. Anesth Analg 92(1):189–193
Magnano D, Montalbano R, Lamarra M, Ferri F, Lorini L, Clarizia S, Rescigno G (2005) Ineffectiveness of local wound anesthesia to reduce postoperative pain after median sternotomy. J Card Surg 20(4):314–318.
Kushner DM, LaGalbo R, Connor JP, Chappell R, Stewart SL, Hartenbach EM (2005) Use of a bupivacaine continuous wound infusion system in gynecologic oncology: a randomized trial. Obstet Gynecol 106(2):227–233.
Kainu JP, Sarvela J, Halonen P, Puro H, Toivonen HJ, Halmesmaki E, Korttila KT (2012) Continuous wound infusion with ropivacaine fails to provide adequate analgesia after caesarean section. Int J Obstet Anesth 21(2):119–124. doi:10.1016/j.ijoa.2011.12.009
Liu FF, Liu XM, Liu XY, Tang J, Jin L, Li WY, Zhang LD (2015) Postoperative continuous wound infusion of ropivacaine has comparable analgesic effects and fewer complications as compared to traditional patient-controlled analgesia with sufentanil in patients undergoing non-cardiac thoracotomy. Int J Clin Exp Med 8(4):5438–5445
Becker DE (2010) Nausea, vomiting, and hiccups: a review of mechanisms and treatment. Anesth Prog 57(4):150–156. doi:10.2344/0003-3006-57.4.150
Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N (2011) Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth 106(3):292–297. doi:10.1093/bja/aeq406
Evans MS, Lysakowski C, Tramer MR (2008) Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth 101(5):610–617
Acknowledgments
Assistance: the authors wish to thank Celine Genty and Kristina Skaare (Clinical Research Centre, INSERM 003, Hôpital Michallon, Grenoble) for their assistance in the statistical analysis, and Marie Joyeux-Faure (Department of Pharmacy, Hôpital Michallon, Grenoble) for her help in preparing blinded treatments. We thank also Dr. Marie Frost (Department of Anaesthesiology and Critical Care, Hôpital Michallon, Grenoble) for her assistance in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support and sponsorship
This work was supported by institutional funding from the Delegation à la Recherche Clinique et à l’Innovation (DRCI), University Grenoble Hospital.
Conflict of interest
None.
Additional information
Jules Greze and Arnaud Vighetti contributed equally to the work.
Rights and permissions
About this article
Cite this article
Greze, J., Vighetti, A., Incagnoli, P. et al. Does continuous wound infiltration enhance baseline intravenous multimodal analgesia after posterior spinal fusion surgery? A randomized, double-blinded, placebo-controlled study. Eur Spine J 26, 832–839 (2017). https://doi.org/10.1007/s00586-016-4428-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4428-1