Abstract
Matrix metalloproteinases (MMPs) regulate connective tissue architecture and cell migration through extracellular matrix (ECM) degradation and are associated with both physiological and pathological processes. Although they are known to play a role in skeletal development, little is known about the role of MMPs in intervertebral disc (IVD) development. Sixteen fetal human lumbar spine segments, obtained at autopsy, were compared with five normal, non-fetal L4–L5 IVDs. Intensity and/or localization of immunohistochemical staining for MMP-1, -2, -3 and -14 were evaluated by three independent observers. MMP-2 production and activation was quantified by gelatin zymography. MMP-1 and -14 were abundantly present in the nucleus pulposus (NP) and notochordal (NC) cells of the fetal IVDs. In non-fetal IVDs, MMP-1 and -14 staining was significantly less intense (p = 0.001 and p < 0.001, respectively). MMP-3 was found in almost the entire IVD with no significant difference from non-fetal IVDs. MMP-2 staining in the NC and NP cells of the fetal IVD was moderate, but weak in the non-fetal IVD. Gelatin zymography showed a negative correlation of age with MMP-2 activity (p < 0.001). MMP-14 immunostaining correlated positively with MMP-2 activity (p = 0.001). For the first time, the presence of MMP-1, -2, -3 and -14 in the fetal human IVD is shown and the high levels of MMP-1, -2 and -14 suggest a role in the development of the IVD. In particular, the gradual decrease in MMP-2 activation during gestation pinpoints this enzyme as key player in fetal development, possibly through activation by MMP-1 and -14.
Similar content being viewed by others
Introduction
Since the identification of the first matrix metalloproteinase (MMPs) in 1962, MMPs have been shown to be associated with a large number of diseases, including vascular, renal, neurological, respiratory, oncological, rheumatic and orthopedic diseases [1, 2]. However, the development of MMP knock-out mice with aberrant phenotypes and the severe side effects of MMP inhibitors in the late 1990s clearly indicated that MMPs are also crucial for normal tissue function [1, 2]. MMPs are associated with complex regulatory processes such as cell migration, tissue architecture and development [2, 3]. Fetal development of connective tissues has also been suggested to be dependent on this class of enzymes and various MMPs have been shown to play a role in skeletal development.
Various MMPs have been shown to be involved in the development of joints. MMP-1 was found in chondrocytes and synovium of fetal human joints [4]. Moreover, MMP-1 activity was found to be greatly increased in synovial fluid of fetal horses compared to juvenile and mature animals [5]. MMP-2 is associated with development of the osteocytic canalicular network, synovial tissue and articular surfaces [4, 6]. In humans, a mutation in the gene encoding for MMP-2 results in a multicentre osteolysis and arthritis syndrome, which is characterized by short stature, facial anomalies, and arthropathy [7]. MMP-3 has been associated with the development of fetal joints and of joint cavities. An increase in MMP-3 activity was found in the synovial fluid of fetal equine joints compared to juvenile and mature horses [4, 8]. The most severe phenotypical abnormalities of all MMP-deficient animals are seen in the MMP-14 knock-out mouse, characterized by dwarfism, osteopenia, arthritis, angiogenesis defects and death within 3–12 weeks after birth [9]. Remodeling of fetal unmineralized cartilage into joints and bone has been shown to be MMP-14-mediated [10].
However, nothing is known about the involvement of MMPs in fetal development of the intervertebral disc (IVD). As the extracellular matrix (ECM) of both articular cartilage and the nucleus pulposus (NP) of the IVD is mainly composed of aggrecan and collagen type II, and the respective degenerative processes have been shown to share many factors, it is likely that also during fetal development, cartilage and IVD tissue share the same mediators [11].
Given this clear association of MMP-1, -2, -3 and -14 with the fetal development of cartilage, the aim of this study was to evaluate their presence and in particular the activity of MMP-2 in human IVDs during consecutive stages of fetal development and compare these to normal non-fetal IVDs.
Materials and methods
Reagents, antibodies and tissue processing
Used reagents, antibodies, antibody concentrations, antigen retrieval and tissue processing are described in the electronic supplement.
Sample acquisition
Fetal IVDs were obtained during a standard post-mortem procedure in which the lumbar spine is partly removed for diagnostic purposes. IVDs from 16 deceased patients (spontaneous abortions, gestation age 15.5–40.3 weeks) were obtained within 24 h after death (supplemental Table 1). Patients with skeletal pathology were excluded from this study. Several lumbar segments, including the vertebrae and adjacent endplates, were obtained. A control group of five non-fetal, non-degenerative, human L4–L5 IVDs (age 3.3–21.8 years) was collected in a similar manner as the fetal IVDs. This control group will be referred to as the non-fetal discs. The scientific committee from the Department of Pathology of the UMC Utrecht approved the study and the material was used in line with the code “Proper Secondary Use of Human Tissue” as installed by the Federation of Biomedical Scientific Societies.
After resection, the discs were cut (fetal discs) or sawed (non-fetal discs) in sagittal samples. Of each IVD, a midsagittal sample was fixed and stored in 4% formalin for histology. The remaining sagittal slices were embedded in TissueTek (Sakura Finetek Europe, Zoeterwoude, The Netherlands), stored at −80°C and were used for protein extraction.
Immunolocalization of MMP-1, -2, -3, -14, collagen type II, endothelial membrane antigen (EMA) and pan cytokeratin (AE1/AE3)
Paraffin sections were rehydrated through xylene and graded ethanol series. Endogenous peroxidase was inactivated by incubation in 0.3% H2O2 in PBS for 15 min, followed by a 20-min block step with PBS/3% BSA. Sections were incubated with primary antibodies and the appropriate secondary antibody. DAB (0.06%) in PBS was used for antigen detection and sections were counterstained with Mayer’s hematoxylin. Negative controls were incubated with isotype controls at the same IgG concentration.
Immunohistochemical staining for MMP-1, -2, -3 and -14 was graded by three independent observers according a semiquantitative four-point scoring scale [12]. An intense staining for the antigen in >50% of the cells was scored as ++, an intense staining in 10–50% of the cells was scored +, ± was scored if the staining was seen in less than 10% of the cells and − was scored when no staining was observed. In each section, the cartilage endplate (CE), NP, AF, and notochordal (NC) cells were analyzed. Scores of the three observers were averaged and outliers, i.e. more than 1 grade difference, were re-evaluated at a consensus meeting.
Collagen type II immunohistochemistry was used to demonstrate the cartilaginous nature of the ECM throughout the fetal and non-fetal IVDs (Supplemental Fig. 2e, f). The EMA and pan cytokeratin (AE1/AE3) staining was used for identification of NC cells [13, 14].
Gelatin zymography for MMP-2 activation and production
Five micrograms of protein of tissue extract was separated on an 8% acrylamide/0.1% gelatin gel. After electrophoresis, the gel was washed twice for 15 min in 2.5% Triton X-100, 50 mM Tris and 10 mM CaCl (pH 7.4) and incubated for 18 h in 0.05% Brij 35, 50 mM Tris, 10 mM CaCl (pH 7.4) at 37°C. Gels were stained with Coomassie Brilliant Blue, destained in deionized water and analyzed by densitometry using the Gel doc 2000 system (Bio-rad, Hercules, CA) and Quantity One software (Bio-rad). A degenerative IVD sample containing active and pro-MMP-2 was used as control sample. Densitometry data were corrected to the measurements of the control sample on each gel. Gelatin zymography of both fetal and non-fetal samples is shown in supplemental Fig. 3. Casein zymography for MMP-1 and MMP-3 was also attempted; however, this technique was not sensitive enough to detect MMP-1 or MMP-3 activity in fetal and non-fetal IVDs.
Statistical analysis
Statistical analysis was performed with SPSS 12.0.1 software. Differences in enzyme content between fetal and non-fetal IVDs were determined by a Mann–Whitney U test. Differences between age groups were analyzed by Kruskal–Wallis’ non-parametric test followed by non-parametric multiple comparison test and Bonferroni correction. Spearman’s non-parametric test was used for correlation analyses. Categorical data obtained by grading of the immunochemical staining were analyzed with the 2 × C Fisher exact test [15].
Results
Immunohistochemistry
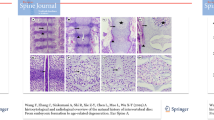
In the non-fetal IVDs, NC cells, identified by their morphology of large irregularly shaped cells containing vesicle-like structures predominantly laying in cell clusters (Fig. 1a) [16, 17], were found to stain positive for EMA and pan cytokeratin (AE1/AE3), which did not stain any other cell type (Fig. 1b, c, respectively). Abundant staining for MMP-1 was seen in the NP of the fetal IVD, whereas staining of the AF and CE was less intense (Fig. 2a). In the fetal NP, both the chondrocyte-like NP cells and the NC cells stained positive for MMP-1(Fig. 2a). In the non-fetal IVDs, MMP-1 staining was moderately positive in NP and AF, while no staining was seen in the CE (Fig. 2b). The positive staining in the NP was mainly localized in the NC cells (Fig. 2b). The intensity of the immunohistochemical staining in all three areas of the IVD (CE, AF and NP) combined was significantly higher in the fetal IVDs compared to the non-fetal IVDs, p = 0.001 (Fig. 3). MMP-2 staining was moderate in the NP, in both chondrocyte-like NP and NC cells, of the fetal IVDs, whereas no staining was found in the CE and AF (Fig. 2c). MMP-2 staining in the non-fetal IVDs was variable throughout the IVD, with in general a moderate to weak staining in the entire IVD (Fig. 2d).
Immunohistochemical staining for MMP-1 (a, b), MMP-2 (c, d), MMP-3 (e, f) and MMP-14 (g, h) in the nucleus pulposus of fetal (a, c, e, g) and non-fetal IVDs (b, d, f, h). Arrows accompanied by “NC” indicate clusters of notochordal cells and within the nucleus pulposus and “NP” indicates the nucleus pulposus containing chondrocyte-like NP cells. Scale bars represent 100 μm (a, b, e, f), 50 μm (g, h) and 25 μm (c, d)
Intensity of immunohistochemical staining for MMP-1, -2, -3 and -14 in fetal and non-fetal IVDs. An intense staining for the antigen in >50% of the cells was scored as ++, an intense staining in 10–50% of the cells was scored +, ± was scored if the staining was seen in less than 10% of the cells and − was scored when no staining was observed, **p < 0.005
MMP-3 was clearly seen in the NP, AF and CE of fetal IVDs (Fig. 2e). Intense staining of the NC cells and a moderate staining of chondrocyte-like NP cells were seen in the NP (Fig. 2e). In the non-fetal IVDs, MMP-3 staining was seen in the NP and moderately in the CE and AF. In the NP the NC cells showed a clear staining and the chondrocyte-like NP cells showed a moderate staining for MMP-3 (Fig. 2f).
The intensity of MMP-14 staining in the fetal IVDs was strong in the NP and moderate in the AF and CE. In the NP, both the NC and chondrocyte-like NP cells stained positive for MMP-14 (Fig. 2g). In the non-fetal IVDs, the NP showed a moderate staining and the AF and CE were negative (Fig. 2h). In the NP, the NC cells were still clearly positive for MMP-14, but a few cartilage-like NP cells were also positive for MMP-14 (Fig. 2h). The intensity of the staining in all three areas of the non-fetal IVDs combined was significantly less than in the fetal IVDs, p ≤ 0.001 (Fig. 3).
Gelatin zymography for MMP-2 activation and production
Gelatin zymography showed a statistically significant negative correlation between age in weeks and active MMP-2 [p < 0.001; correlation coefficient (CC) −0.80]. Also with respect to fetal development only (first 3 age groups), a negative correlation was found for MMP-2 activity and gestational age (p = 0.013 CC = −0.61). No correlation was found between levels of pro-MMP-2 and age. The level of active MMP-2 in the fetal groups was higher in comparison to the non-fetal discs (p = 0.004). Also the levels of pro-MMP-2 were significantly higher in fetal samples compared to non-fetal discs (p = 0.008) (Fig. 4).
A significant decrease in active MMP-2 was found between the 15–22 weeks after gestation group and the non-fetal group (0–22 years, p < 0.001). The level of pro-MMP-2 was significantly higher in 30–41 weeks after gestation group, compared to the IVDs from the non-fetal group (0-22 years, p = 0.044). Active MMP-2 levels in all IVD samples showed a strong positive correlation with the intensity of MMP-14 immunohistochemistry (p = 0.001, CC = 0.673). Faint MMP-9 bands were found in most samples, no differences in active and pro-MMP-9 levels were seen between fetal and non-fetal IVDs.
Discussion
This study for the first time shows the presence of MMP-1, -2, -3 and -14 in fetal and non-fetal human IVDs. The enzymes were mainly localized in the NP and in the NC cells of the IVD. Significantly, more MMP-1 and -14 were seen in the fetal IVDs compared to the non-fetal IVDs. Moreover, MMP-2 activity during fetal development was high during the first 15–22 weeks of gestation and decreased with gestational age and even further in postnatal and young healthy IVDs.
Not much is known about the presence of MMPs in fetal and non-fetal IVDs. An immunohistochemical study describing the presence of MMP-1, -2, -3 and -9 in degenerative human IVDs included some data on an unknown number of fetal and non-fetal IVDs of donors of undefined age [18]. In contrast to the present data, these MMPs were not found in fetal IVDs and only an occasional staining for MMP-1, -2 and -3 was reported in non-degenerative IVDs [18]. The discrepancies with this study could be explained by various factors such as different and less sensitive immunohistochemistry protocols and the limited number of fetal and non-degenerative IVDs in the degenerative disc study.
In this study, it was shown that these enzymes are present in the fetal IVD and that their levels decreased during childhood and adolescence, suggesting a physiological function of these enzymes during development of the IVD. In support of this role is the available literature on MMP-1, -2, -3 and -14, clearly associating these enzymes with the development of connective tissues, including cartilaginous tissue. The function of these MMPs during the fetal development of the IVD could be: direct ECM degradation for cell migration or tissue remodeling, regulation of the activity of other proteases by removal of the pro-domain and modulation of biologically active molecules by direct cleavage or release from ECM store [19]. MMP-14 is the only MMP, which has specifically been studied in non-degenerative IVDs. A rat IVD organ culture study describes the presence of MMP-14 in chondrocytes migrating from the CE to the NP and an extensive immunohistochemical positivity of the NC cells for MMP-14 is reported [20], in concordance with the current results. The authors suggest that MMP-14 enables the chondrocytes to migrate from the CE to the NP and replace the NC cells [20]. During growth of the IVD, the normal ECM has to be degraded to allow IVD cells to migrate through the tissue [21]. Additionally, existing ECM could undergo partial or complete degradation prior to new matrix synthesis [21]. MMP-1, -2, -3 and -14 are all capable of degrading one or both of the most important ECM components of the fetal IVD, collagen type II and aggrecan.
Besides their role in remodeling by ECM degradation, several MMPs also have regulatory functions by activating other proteases [2]. In the developing IVD, MMP-1 and -14 could have a regulatory function since both enzymes are capable of activating several MMPs including MMP-2 [22]. Additionally, regulatory activity of MMP-1 could be regulated by MMP-3 which is capable of not only activating pro-MMP-1 but also pro-MMP-7, -8, -9 and -13 [22]. The strongly abnormal phenotype of several MMP knock-out mice may well be caused by these important regulatory functions of the enzymes. In particular, MMP-14 knock-out mice display decreased proliferation of chondrocytes in growth plates and defective vascular invasion of cartilage ends of fetal long bones, which leads to shortening and skeletal malformation [9, 23]. The decreased activation of MMP-2 in MMP-14-deficient mice suggests that the function of MMP-14 during development may not just be limited to ECM turnover and that MMP-14 also has a more regulatory function [23]. The statistically significant correlation between MMP-2 activity and the intensity of MMP-14 immunohistochemistry could be an indication that MMP-2 activity in fetal IVDs is regulated by MMP-14.
Strikingly, MMP-14 and -1 were clearly present in the NC cells of the fetal IVD, suggesting a possible role for these cells in the regulation of extracellular remodeling and cell migration. This theory is supported by the fact that the loss of these cells during late childhood or adolescence is associated with the onset of IVD degeneration [24]. Moreover, recently rat NC cells have been found to stimulate migration of rat CE chondrocytes in vitro [25]. NC cells are remnants of the embryonic notochord and are entrapped inside the IVD during embryonic formation of the vertebrae and IVDs [17]. There are two hypotheses on the role of NC cells during IVD development. The first postulates that NC cells regulate matrix synthesis and cell migration and undergo apoptosis once disc formation is complete. The second theory assumes that NC cells directly synthesize the ECM of the NP and then differentiate into chondrocyte-like NP cells [17]. Since the NC cells were found to stain less intense for proteoglycans than the chondrocyte-like NP cells and hardly any staining for collagen type II is seen in the ECM surrounding the NC cells, the first theory seems to be most likely.
Although this study clearly shows the presence of MMP-1, -2, -3 and -14 in the fetal IVD, it must be kept in mind that immunohistochemistry is seldom capable of distinguishing between active and inactive forms of enzymes. Only the actual activity of MMP-2 could be determined here, whereas casein zymography and ELISA-based activity assays were not sensitive enough to measure detectable levels of enzyme activity. Although PCR is a very sensitive technique, the presence mRNA levels do not always correlate to the protein levels and PCR is unable to detect the presence of active MMPs. More sensitive techniques determining actual MMP activity are required to further elucidate the role of MMPs in IVD development. As to the clear association of IVD development with MMP-2 activity, it remains unknown how MMP-2 exactly is involved in IVD, or even cartilage or joint development. A role in ECM turnover and growth seems likely; however, no support for this hypothesis can be found in literature. It is remarkable that MMP-2 seems to have such a close association with the development of connective tissues and is also strongly associated with degenerative diseases as osteoarthritis and IVD degeneration [26]. MMP knock-out mice studies could further elucidate the role of MMPs in fetal IVD development; however, results of these studies should be analyzed with care since human and mice MMPs are not entirely comparable.
In addition to the MMPs studied, other proteases may be involved in fetal development of the IVD. However, the enzymes currently studied are the only MMPs shown to be involved in the development of cartilage and joints. ADAMTS-4 and -5 may provide good candidates for IVD development through ECM turnover. Nevertheless, neither of these enzymes have been shown to be required for normal cartilage and skeletal development as demonstrated in knock-out models [27].
Conclusion
This study clearly shows the presence of MMP-1, -2, -3 and -14 in the fetal human IVD. Their increased expression as compared to non-fetal healthy IVDs suggests their involvement in IVD development. In particular, MMP-2 activity was closely correlated to developmental stage. During IVD development, MMPs could be involved in cell migration, regulation of ECM turnover and activation of other MMPs.
References
Le Maitre CL, Freemont AJ, Hoyland JA (2004) Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol 204:47–54
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8:221–233
Lemaitre V, D’armiento J (2006) Matrix metalloproteinases in development, disease. Birth Defects Res C Embryo Today 78:1–10
Edwards JC, Wilkinson LS, Soothill P, Hembry RM, Murphy G, Reynolds JJ (1996) Matrix metalloproteinases in the formation of human synovial joint cavities. J Anat 188:355–360
Brama PA, van den Boom R, de Groot J, Kiers GH, van Weeren PR (2004) Collagenase-1 (MMP-1) activity in equine synovial fluid: influence of age, joint pathology, exercise and repeated arthrocentesis. Equine Vet J 36:34–40
Inoue K, Mikuni-Takagaki Y, Oikawa K, Itoh T, Inada M, Noguchi T et al (2006) A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem 281:33814–33824
Martignetti JA, Al Aqeel A, Sewaira WA, Boumah CE, Kambouris M, Mayouf SA et al (2001) Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet 28:261–265
Brama PA, TeKoppele JM, Beekman B, van Ei B, Barneveld A, van Weeren PR (2000) Influence of development and joint pathology on stromelysin enzyme activity in equine synovial fluid. Ann Rheum Dis 59:155–157
Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA et al (1999) MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99:81–92
Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H (2003) MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J Cell Biol 163:661–671
Roberts S, Evans H, Trividi J, Menage J (2006) Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 88(S2):10–14
Zlobec I, Steele R, Michel RP, Compton CC, Lugli A, Jass JR (2006) Scoring of p53, VEGF, Bcl-2 and APAF-1 immunohistochemistry and interobserver reliability in colorectal cancer. Mod Pathol 19:1236–1242
Chordoma MM (1984) Antibodies to epithelial membrane antigen and carcinoembryonic antigen in differential diagnosis. Arch Pathol Lab Med 108:891–892
Naka T, Iwamoto Y, Shinohara N, Chuman H, Fukui M, Tsuneyoshi M (1997) Cytokeratin subtyping in chordomas and the fetal notochord: and immunohistochemical analysis of aberrant expression. Mod Pathol 10:545–551.15
Requena F, Ciudad NM (2006) A major improvement to the network algorithm for Fisher’s Exact Test in 2xc contingency tables. Comput Stat Data Anal 51:490–498
Guehring T, Urban JP, Cui Z, Tirlapur UK (2008) Noninvasive 3D vital imaging and characterization of notochordal cells of the intervertebral disc by femtosecond near-infrared two-photon laser scanning microscopy and spatial-volume rendering. Microsc Res Technol 71:298–304
Hunter CJ, Matyas JR, Duncan NA (2003) The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng 9:667–677
Weiler C, Nerlich A, Zipperer J, Bachmeier BE, Boos N (2002) 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J 11:308–320
Ortega N, Behonick D, Stickens D, Werb Z (2003) How proteases regulate bone morphogenesis. Ann N Y Acad Sci 995:109–116
Kim KW, Ha KY, Park JB, Woo YK, Chung HN, An HS (2005) Expression of membrane-type I matrix metalloproteinase, Ki-67 protein, and type II collagen by chondrocytes migrating from cartilage endplate into nucleus pulposus in rat intervertebral disc. Spine 30:1373–1378
Holmbeck K, Szabova L (2006) Aspects of extracellular matrix remodeling in development, disease. Birth Defects Res C Embryo Today 78:11–23
Murphy G, Knauper V, Atkinson S, Butler G, Hutton M, Starcke J et al (2002) Matrix metalloproteinases in arthritic disease. Arthritis Res 4(S3):39–49
Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rause RW et al (2000) Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci 97:4052–4057
Aguiar DJ, Johnson SL, Oegema TR (1999) Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res 246:129–137
Kim KW, Ha KY, Lee JS, Nam SW, Woo YK, Lim TH et al (2009) Notochordal cells stimulate migration of cartilage endplate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine 9:323–329
Rutges J, Kummer J, Oner F, Verbout A, Castelein R, Roestenbrug H et al (2008) Increased MMP-2 activity during intervertebral disc degeneration is correlated to MMP-14 levels. J Pathol 214:523–530
Rogerson FM, Stanton H, East CJ, Golub SB, Tutolo L, Farmer PJ et al (2008) Evidence of a novel aggrecan-degrading activity in cartilage: studies of mice deficient in both ADAMTS-4 and ADAMTS-5. Arthritis Rheum 58:1664–1673
Acknowledgments
The authors acknowledge F. Bernhard and A. de Ruiter from the Department of Pathology of the University Medical Center Utrecht for their help in obtaining the IVD specimens. This study was supported by the Dutch Arthritis Association and the Anna Foundation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rutges, J.P.H.J., Nikkels, P.G.J., Oner, F.C. et al. The presence of extracellular matrix degrading metalloproteinases during fetal development of the intervertebral disc. Eur Spine J 19, 1340–1346 (2010). https://doi.org/10.1007/s00586-010-1378-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-010-1378-x