Abstract
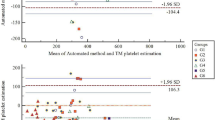
In the present study, the LaserCyte instrument, a fully automated flow cytometer for use in veterinary practice, was evaluated for dogs and cats. Precision (coefficient of variation, CV) for red blood cell (RBC) parameters was ≤3.9%, for reticulocytes between 14.9 and 102%, for white blood cells (WBC) between 3 and 9.5%, for neutrophils between 3.9 and 6.5%, for lymphocytes between 7 and 17.9%, for monocytes between 4.9 and 13.1%, for eosinophils between 10.4 and 32.1%, for basophils between 7.8 and 32%, for platelets between 3.1 and 13.2%, and for platelet indices between 0 and 28.2%. The range of linearity extended the reference ranges. The agreement with reference methods (coefficient of correlation, r) were ≥0.96 (RBC), ≥0.94 (hematocrit), ≥0.96 (hemoglobin), ≥0.95 (mean corpuscular volume), ≥0.94 (WBC), ≥0.93 (neutrophils), ≥0.77 (lymphocytes), ≥0.77 (monocytes), ≥0.29 (eosinophils), ≥0.03 (basophils), ≥0.13 (reticulocytes), and ≥0.86 (platelets). The LaserCyte allowed the correct assessment of RBC and WBC parameters with respect to clinical relevance in the majority of samples. Lymphocytopenia was detected in only 51 out of 89 cases and monocytopenia in one out of 11 cases. The reticulocyte counts were correctly estimated in 85 out of 149 cases. It was concluded that the LaserCyte allowed reliable determination of the RBC parameters, WBCs, neutrophils in both species and platelets in dogs. Based on its capability to reliably determine feline platelets and of the parameters mentioned above, this instrument is considered a useful in-house analyzer for the veterinary practice. Qualitative microscopic assessment of blood smears is still necessary for detecting abnormal cell morphologies, certain cell precursors and blood parasites.




Similar content being viewed by others

Notes
Micro tube K3-EDTA, Sarstedt, D-51588 Nümbrecht.
BD Vacutainer, No Additive, Franklin Lakes, NJ 07417-1885, USA.
Abbott AG, Baar, Switzerland.
Hema Tek 1000, Bayer AG, Zürich, Switzerland.
Assistant Germany, Karl Hecht AG, D-97647 Sandheim.
Becton Dickinson and Company, Ranklin Lakes NJ 07414-1885, USA.
Hettich Centrifuge, Rotanta 460 S, 8806 Bäch, Switzerland.
Dulbecco’s phosphate buffered saline, SIGMA-Aldrich Company, LTD Ir vine, Ayrshire KA 12 8NB, UK.
Excel 2003, Microsoft Corporation, 1 Microsoft Way, Redmond, WA 98052-6399, USA.
Stat View software, Version 5, SAS Campus Drive, Cary, NC 27513-2414, USA.
References
Altman DG (1994) Practical statistic for medical research. Chapman & Hall, London, UK
Bablok W, Passing H (1985) Application of statistical procedures in analytical instrument testing. J Clin Lab Autom 7:74–79
Bablok W, Passing H, Bender R, Schneider B (1988) A general regression procedure for method transformation. Application of linear regression procedures for method comparison studies in clinical chemistry, Part III. J Clin Chem Clin Biochem 26:783–790
Bienzle D, Stanton JB, Embry JM, Bush SE, Mahaffey EA (2000) Evaluation of an in-house centrifugal hematology analyzer for use in veterinary practice. J Am Vet Med Assoc 217:1195–1200
Bollinger PB, Drewinko B, Brailas CD, Smeeton NA, Trujillo JM (1987) The technicon H*1—an automated hematology analyzer for today and tomorrow. Am J Clin Pathol 87:71–78
Eisenwiener HG, Bablok W, Bardorff W, Bender R, Markowetz D, Passing H, Spaethe R, Specht W, Völkert E (1984) Statistische Auswertung beim Methodenvergleich. Lab Med 8:232–244
Feldman BV, Zinkl JG, Jain NC (2000) Schalm’s veterinary hematology. Lippincott Williams & Wilkins, Philadelphia, PA, pp 1058–1065
Fulwyler MJ (1980) Flow cytometry and cell sorting. Blood Cells 6:173–184
Hofmann-Lehmann R, Wegmann D, Winkler G, Lutz H (1998) Evaluation of the QBC-vet autoread hematology system for domestic and pet animal species. Comp Haematol Int 8:108–116
Jensen AL (2000) Validation of diagnostic tests in hematology laboratories. Lippincott Williams & Wilkins, Philadelphia, PA
Kieffer J, Winkler G, Van Hove L, Walsh A, Thomann P, Wyss S, Eggenberger E, Lutz H (1999) Evaluation of the CELL-DYN 3500 hematology instrument for the analysis of the mouse and rat blood. Comp Haematol Int 9:92–106
Knoll JS, Rowell SL (1996) Clinical hematology: in-clinic analysis, quality control, reference values, and system selection. Vet Clin North Am Small Anim Pract 26:981–1002
Norman EJ, Barron RCJ, N Andrew S, Clampitt RB (2001) Prevalence of low automated platelet counts in cats: comparison with prevalence of thrombocytopenia based on blood smear estimation. Vet Clin Pathol 30:137–140
Passing H, Bablok W (1983) A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem 21:709–720
Passing H, Bablok W (1984) Comparison of several regression procedures for method comparison studies and determination of sample sizes. Application of linear regression procedures for method comparison studies in clinical chemistry, Part II. J Clin Chem Clin Biochem 22:431–445
Passing H, Bablok W, Glocke M (1981) An optimized design for the establishment of assigned values in control sera. The establishment of assigned values in control sera, IV. J Clin Chem Clin Biochem 19:1167–1179
Perkins PC, Grindem CB, Cullins LD (1995) Flow cytometric analysis of punctate and aggregate reticulocyte responses in phlebotomized cats. Am J Vet Res 56:1564–1569
Petrie A, Watson P (1999) Statistics for veterinary and animal science. Blackwell, Oxford, UK
Pohland D (1989) Evaluation of the automated hematology analyser sysmex M-2000. J Clin Chem Clin Biochem 27:41–47
Sachse C, Henkel E (1996) An evaluation of the CELL-DYN 1700 hematology analyser: automated cell counting and three-part leukocyte differentiation. Clin Lab Haematol 18:171–180
Tisdall PA (1985) Evaluation of a laser-based three-part leukocyte differential analyzer in detection of clinical abnormalities. Lab Med 16:228–233
Tvedten H, Korcal D (1996) Automated differential leukocyte count in horses, cattle, and cats using the technicon H-1E hematology system. Vet Clin Pathol 25:14–22
Tvedten HW, Wilkins RJ (1988) Automated blood cell counting systems: a comparison of the Coulter S-Plus IV, Ortho ELT-8/DS, Ortho ELT-8/WS, Technicon H-1, and Sysmex E-5,000. Vet Clin Pathol 17:47–54
Winkler GC, Engeli E, Rogg E, Kieffer J, Kellenberger H, Lutz H (1995) Evaluation of the Contraves AL 820 automated hematology analyser for domestic, pet and laboratory animals. Comp Haematol Int 5:130–139
Acknowledgements
We thank the technicians of the Clinical Laboratory, Vetsuisse Faculty, University of Zurich, and Yvonne Sigrist for excellent support throughout the project. Thanks are also due to our colleagues in the clinics for providing the blood samples, and to Brigitte Egg and Richard Andrews for technical support. Regina Hofmann-Lehmann is the recipient of a professorship by the Swiss National Science Foundation (PP00B-102866). Sources of funding: IDEXX Laboratories, 1 IDEXX Drive, Westbrook, Maine 04092, U.S.A.
Author information
Authors and Affiliations
Corresponding author
Additional information
IDEXX Laboratories, Westbrook, Maine 04092, USA.
Rights and permissions
About this article
Cite this article
Wenger-Riggenbach, B., Hässig, M., Hofmann-Lehmann, R. et al. Evaluation of the LaserCyte: an in-house hematology analyzer for dogs and cats. Comp Clin Pathol 15, 117–129 (2006). https://doi.org/10.1007/s00580-006-0602-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-006-0602-x