Abstract
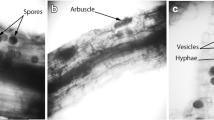
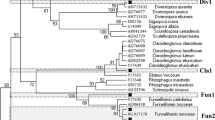
Variation in the abiotic environment and host plant preferences can affect the composition of arbuscular mycorrhizal (AMF) assemblages. This study analyzed the AMF taxa present in soil and seedlings of Artemisia tridentata ssp. wyomingensis collected from sagebrush steppe communities in southwestern Idaho, USA. Our aims were to determine the AMF diversity within and among these communities and the extent to which preferential AMF–plant associations develop during seedling establishment. Mycorrhizae were identified using molecular methods following DNA extraction from field and pot culture samples. The extracted DNA was amplified using Glomeromycota specific primers, and identification of AMF was based on phylogenetic analysis of sequences from the large subunit-D2 rDNA region. The phylogenetic analyses revealed seven phylotypes, two within the Claroideoglomeraceae and five within the Glomeraceae. Four phylotypes clustered with known species including Claroideoglomus claroideum, Rhizophagus irregularis, Glomus microaggregatum, and Funneliformis mosseae. The other three phylotypes were similar to several published sequences not included in the phylogenetic analysis, but all of these were from uncultured and unnamed glomeromycetes. Pairwise distance analysis revealed some phylotypes with high genetic variation. The most diverse was the phylotype that included R. irregularis, which contained sequences showing pairwise differences up to 12 %. Most of the diversity in AMF sequences occurred within sites. The smaller genetic differentiation detected among sites was correlated with differences in soil texture. In addition, multiplication in pot cultures led to differentiation of AMF communities. Comparison of sequences obtained from the soil with those from A. tridentata roots revealed no significant differences between the AMF present in these samples. Overall, the sites sampled were dominated by cosmopolitan AMF taxa, and young seedlings of A. tridentata ssp. wyomingensis were colonized in relation to the abundance of these taxa in the soil.




Similar content being viewed by others
References
Allen M, Richards J, Busso C (1989) Influence of clipping and soil water status on vesicular-arbuscular mycorrhizae of two semi-arid tussock grasses. Biol Fertil Soils 8:285–289
Appoloni S, Lekberg Y, Tercek MT, Zabinski CA, Redecker D (2008) Molecular community analysis of arbuscular mycorrhizal fungi in roots of geothermal soils in Yellowstone National Park (USA). Microb Ecol 56:649–659
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Barea JM, Palenzuela J, Cornejo P, Sánchez-Castro I, Navarro-Fernández C, Lopéz-Garcia A, Estrada B, Azcón R, Ferrol N, Azcón-Aguilar C (2011) Ecological and functional roles of mycorrhizas in semi-arid ecosystems of Southeast Spain. J Arid Environ 75:1292–1301
Börstler B, Raab PA, Thiéry O, Morton JB, Redecker D (2008) Genetic diversity of the arbuscular mycorrhizal fungus Glomus intraradices as determined by mitochondrial large subunit rRNA gene sequences is considerably higher than previously expected. New Phytol 180:452–465
Börstler B, Thiéry O, Sýkorová Z, Berner A, Redecker D (2010) Diversity of mitochondrial large subunit rDNA haplotypes of Glomus intraradices in two agricultural field experiments and two semi-natural grasslands. Mol Ecol 19:1497–1511
Busby RR, Stromberger ME, Rodriguez G, Gebhart DL, Paschke MW (2013) Arbuscular mycorrhizal fungal community differs between a coexisting native shrub and introduced annual grass. Mycorrhiza 23:129–141
Cesaro P, van Tuinen D, Copetta A, Chatagnier O, Berta G, Gianinazzi S, Lingua G (2008) Preferential colonization of Solanum tuberosum L. roots by the fungus Glomus intraradices in arable soil of a potato farming area. Appl Environ Microbiol 74:5776–5783
Croll D, Wille L, Gamper HA, Mathimaran N, Lammers PJ, Corradi N, Sanders IR (2008) Genetic diversity and host plant preferences revealed by simple sequence repeat and mitochondrial markers in a population of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 178:672–687
del Mar AM, Torrecillas E, Torres P, Garcia-Orenes F, Roldán A (2012) Long-term effects of irrigation with waste water on soil AM fungi diversity and microbial activities: the implications for agro-ecosystem resilience. Plos One 7
Egerton-Warburton LM, Allen EB (2000) Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol Appl 10:484–496
Evans DG, Miller MH (1990) The role of the external mycelial network in the effect of soil disturbance upon vesicular arbuscular mycorrhizal colonization of maize. New Phytol 114:65–71
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes—application to human mitochondrial-DNA restriction data. Genetics 131:479–491
Farris JS, Kallersjo M, Kluge AG, Bult C (1994) Testing significance of incongruence. Cladistics Int J Willi Hennig Soc 10:315–319
Felsenstein J (1985) Confidence-limits of phylogenies—an approach using bootstrap. Evolution 39:783–791
Gollotte A, van Tuinen D, Atkinson D (2004) Diversity of arbuscular mycorrhizal fungi colonising roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza 14:111–117
Graham JH, Leonard RT, Menge JA (1982) Interaction of light intensity and soil temperature with phosphorus inhibition of vesicular-arbuscular mycorrhizae formation. New Phytol 91:683–690
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Helgason T, Merryweather JW, Young JPW, Fitter AH (2007) Specificity and resilience in the arbuscular mycorrhizal fungi of a natural woodland community. J Ecol 95:623–630
Ji B, Bentivenga SP, Casper BB (2012) Comparisons of AM fungal spore communities with the same hosts but different soil chemistries over local and geographic scales. Oecologia 168:187–197
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Johnson NC, Rowland DL, Corkidi L, Allen EB (2008) Characteristics of plant winners and losers in grassland eutrophication—importance of allocation plasticity and mycorrhiza function. Ecology 89:2868–2878
Johnson NC, Wilson GWT, Bowker MA, Wilson J, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci U S A 107:2093–2098
Jones MD, Smith SE (2004) Exploring functional definitions of mycorrhizas: are mycorrhizas always mutualisms? Can J Bot/Rev Can Bot 82:1089–1109
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Buecking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide-sequences. J Mol Evol 16:111–120
Klironomos JN (2000) Host-specificity and functional diversity among arbuscular mycorrhizal fungi. In: Bell CR, Brylinsky M, Johnson-Green P (eds) Microbial biosystems: new frontiers. Atlantic Canada Society for Microbial Ecology, Halifax, pp 845–851
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Kluge AG, Farris JS (1969) Quantitative phyletics and evolution of anurans. Syst Zool 18:1–32
Koch AM, Croll D, Sanders IR (2006) Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecol Lett 9:103–110
Krüger M, Stockinger H, Krüger C, Schüßler A (2009) DNA-based species level detection of Glomeromycota: one PCR primer set for all arbuscular mycorrhizal fungi. New Phytol 183:212–223
Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984
Lambrecht SC, Shattuck AK, Loik ME (2007) Combined drought and episodic freezing effects on seedlings of low‐and high‐elevation subspecies of sagebrush (Artemisia tridentata). Physiol Plant 130:207–217
Li L-F, Li T, Zhang Y, Zhao Z-W (2010) Molecular diversity of arbuscular mycorrhizal fungi and their distribution patterns related to host-plants and habitats in a hot and arid ecosystem, southwest China. FEMS Microbiol Ecol 71:418–427
Liesack W, Weyland H, Stackebrandt E (1991) Potential risks of gene amplification by PCR as determined by 16S rDNA analysis of a mixed-culture of strict barophilic bacteria. Microb Ecol 21:191–198
Lindermann R (2000) Effects of mycorrhizas on plant tolerances to diseases. In: Kapulnik Y, Douds DDJ (eds) Arbuscular mycorrhizas: physiology and function. Kluwer Academic, Dordrecht, pp 173–200
Liu Y, He J, Shi G, An L, Öpik M, Feng H (2011) Diverse communities of arbuscular mycorrhizal fungi inhabit sites with very high altitude in Tibet Plateau. FEMS Microbiol Ecol 78:355–365
Minchin PR (1987) An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 69:89–107
Morton JB, Koske RE, Stürmer SL, Bentivenga SP (2004) Mutualistic arbuscular endomycorrhizal fungi. In: Mueller G, Bills G, Foster M (eds) Biodiversity of fungi: inventory and monitoring methods. Academic Press, San Diego, pp 317–336
Muller K (2004) PRAP-computation of Bremer support for large data sets. Mol Phylogen Evol 31:780–782
Mummey DL, Rillig MC (2006) The invasive plant species Centaurea maculosa alters arbuscular mycorrhizal fungal communities in the field. Plant Soil 288:81–90
Mummey DL, Rillig MC (2007) Evaluation of LSU rRNA-gene PCR primers for analysis of arbuscular mycorrhizal fungal communities via terminal restriction fragment length polymorphism analysis. J Microbiol Methods 70:200–204
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests M (2007) The vegan package. Community ecology package version: 1.8-5
O’Mullan GD, Ward BB (2005) Relationship of temporal and spatial variabilities of ammonia-oxidizing bacteria to nitrification rates in Monterey Bay, California. Appl Environ Microbiol 71:697–705
Öpik M, Moora M, Liira J, Zobel M (2006) Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol 94:778–790
Perryman BL, Maier AM, Hild AL, Olson RA (2001) Demographic characteristics of 3 Artemisia tridentata Nutt. subspecies. J Range Manage 54:166–170
Pivato B, Mazurier S, Lemanceau P, Siblot S, Berta G, Mougel C, van Tuinen D (2007) Medicago species affect the community composition of arbuscular mycorrhizal fungi associated with roots. New Phytol 176:197–210
Poos MS, Jackson DA (2012) Addressing the removal of rare species in multivariate bioassessments: the impact of methodological choices. Ecol Indicators 18:82–90
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Rambaut A, Drummond A (2005) Tracer. A program for analysing results from Bayesian MCMC programs such as BEAST & MrBayes. Version 1.3. Oxford University, [online] http://evolve.zoo.ox.ac.uk/software.html. Accessed 25 April 2013
R Development Core Team (2013) R: a language and environment for statistical computing R Foundation for Statistical Computing. Austria, Vienna
Redecker D, Schüßler A, Stockinger H, Stürmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531
Renker C, Weißhuhn K, Kellner H, Buscot F (2006) Rationalizing molecular analysis of field-collected roots for assessing diversity of arbuscular mycorrhizal fungi: to pool, or not to pool, that is the question. Mycorrhiza 16:525–531
Rosendahl S, Stukenbrock EH (2004) Community structure of arbuscular mycorrhizal fungi in undisturbed vegetation revealed by analyses of LSU rDNA sequences. Mol Ecol 13:3179–3186
Rosendahl S (2008) Communities, populations and individuals of arbuscular mycorrhizal fungi. New Phytol 178:253–266
Sanders IR (2003) Preference, specificity and cheating in the arbuscular mycorrhizal symbiosis. Trends Plant Sci 8:143–145
Schechter SP, Bruns TD (2013) A common garden test of host-symbiont specificity supports a dominant role for soil type in determining AMF assemblage structure in Collinsia sparsiflora. Plos One 8
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421
Schüßler A (2013) Phylogeny and taxonomy of Glomeromycota. http://schuessler.userweb.mwn.de/amphylo/. Accessed 15 June 2013
Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol 62:227–250
Stahl PD, Schuman GE, Frost SM, Williams SE (1998) Arbuscular mycorrhizae and water stress tolerance of Wyoming big sagebrush seedlings. Soil Sci Soc Am J 62:1309–1313
Stockinger H, Krüger M, Schüßler A (2010) DNA barcoding of arbuscular mycorrhizal fungi. New Phytol 187:461–474
Stutz JC, Copeman R, Martin CA, Morton JB (2000) Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southwestern North America and Namibia, Africa. Can J Bot 78:237–245
Swofford D (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates, Sunderland, Massachusetts
Sýkorová Z, Ineichen K, Wiemken A, Redecker D (2007) The cultivation bias: different communities of arbuscular mycorrhizal fungi detected in roots from the field, from bait plants transplanted to the field, and from a greenhouse trap experiment. Mycorrhiza 18:1–14
Torrecillas E, del Mar AM, Roldán A (2012) Differences in the AMF diversity in soil and roots between two annual and perennial gramineous plants co-occurring in a Mediterranean, semiarid degraded area. Plant Soil 354:97–106
van de Voorde TFJ, van der Putten WH, Gamper HA, Hol WHG, Bezemer TM (2010) Comparing arbuscular mycorrhizal communities of individual plants in a grassland biodiversity experiment. New Phytol 186:746–754
van Tuinen D, Jacquot E, Zhao B, Gollotte A, Gianinazzi-Pearson V (1998) Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol Ecol 7:879–887
Vandenkoornhuyse P, Husband R, Daniell TJ, Watson IJ, Duck JM, Fitter AH, Young JPW (2002) Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol Ecol 11:1555–1564
Verbruggen E, Roling WFM, Gamper HA, Kowalchuk GA, Verhoef HA, van der Heijden MGA (2010) Positive effects of organic farming on below-ground mutualists: large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol 186:968–979
Wang Y, Huang Y, Qiu Q, Xin G, Yang Z, Shi S (2011) Flooding greatly affects the diversity of arbuscular mycorrhizal fungi communities in the roots of wetland plants. Plos One 6
Weinbaum BS, Allen MF, Allen EB (1996) Survival of arbuscular mycorrhizal fungi following reciprocal transplanting across the Great Basin, USA. Ecol Appl 6:1365–1372
Acknowledgments
This work was supported by a grant from the U.S. Department of Agriculture-National Institute of Food and Agriculture (grant no. 2010-85101-20480).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carter, K.A., Smith, J.F., White, M.M. et al. Assessing the diversity of arbuscular mycorrhizal fungi in semiarid shrublands dominated by Artemisia tridentata ssp. wyomingensis . Mycorrhiza 24, 301–314 (2014). https://doi.org/10.1007/s00572-013-0537-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-013-0537-4