Abstract
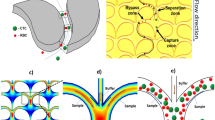
Microsystem-based technologies are providing new opportunities in the area of in vitro diagnostics due to their ability to provide process automation enabling point-of-care operation. As an example, microsystems used for the isolation and analysis of circulating tumor cells (CTCs) from complex, heterogeneous samples in an automated fashion with improved recoveries and selectivity are providing new opportunities for this important biomarker. Unfortunately, many of the existing microfluidic systems lack the throughput capabilities and/or are too expensive to manufacture to warrant their widespread use in clinical testing scenarios. Here, we describe a disposable, all-polymer, microfluidic system for the high-throughput (HT) isolation of CTCs directly from whole blood inputs. The device employs an array of high aspect ratio (HAR), parallel, sinusoidal microchannels (25 × 150 μm; W × D; AR = 6.0) with walls covalently decorated with anti-EpCAM antibodies to provide affinity-based isolation of CTCs. Channel width, which is similar to an average CTC diameter (10–20 μm), plays a critical role in maximizing the probability of cell/wall interactions and allows for achieving high CTC recovery. The extended channel depth allows for increased throughput at the optimized flow velocity (2 mm/s in a microchannel); maximizes cell recovery, and prevents clogging of the microfluidic channels during blood processing. Fluidic addressing of the microchannel array with a minimal device footprint is provided by large cross-sectional area feed and exit channels poised orthogonal to the network of the sinusoidal capillary channels (so-called Z-geometry). Computational modeling was used to confirm uniform addressing of the channels in the isolation bed. Devices with various numbers of parallel microchannels ranging from 50 to 320 have been successfully constructed. Cyclic olefin copolymer (COC) was chosen as the substrate material due to its superior properties during UV-activation of the HAR microchannels surfaces prior to antibody attachment. Operation of the HT-CTC device has been validated by isolation of CTCs directly from blood secured from patients with metastatic prostate cancer. High CTC sample purities (low number of contaminating white blood cells) allowed for direct lysis and molecular profiling of isolated CTCs.







Similar content being viewed by others
References
Adams AA, Okagbare PI, Feng J, Hupert ML, Patterson D, Goettert J, McCarley RL, Nikitopoulos D, Murphy MC, Soper SA (2008) Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. J Am Chem Soc 130(27):8633–8641. doi:10.1021/ja8015022
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20):6897–6904. doi:10.1158/1078-0432.CCR-04-0378
Becker H, Gartner C (2000) Polymer microfabrication methods for microfluidic analytical applications. Electrophoresis 21(1):12–26
Becker H, Heim U (2000) Hot embossing as a method for the fabrication of polymer high aspect ratio structures. Sensor Actuat A Phys 83(1–3):130–135. doi:10.1016/S0924-4247(00)00296-X
Becker H, Locascio LE (2002) Polymer microfluidic devices. Talanta 56(2):267–287
Chang KC, Hammer DA (1999) The forward rate of binding of surface-tethered reactants: effect of relative motion between two surfaces. Biophys J 76(3):1280–1292. doi:10.1016/S0006-3495(99)77291-7
Chin CD, Linder V, Sia SK (2012) Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 12(12):2118–2134
Dharmasiri U, Balamurugan S, Adams AA, Okagbare PI, Obubuafo A, Soper SA (2009) Highly efficient capture and enumeration of low abundance prostate cancer cells using prostate-specific membrane antigen aptamers immobilized to a polymeric microfluidic device. Electrophoresis 30:1–12. doi:10.1002/elps.200900141
Dharmasiri U, Witek MA, Adams AA, Soper SA (2010) Microsystems for the capture of low-abundance cells. Ann Rev Anal Chem 3:409–431. doi:10.1146/annurev.anchem.111808.073610
Dharmasiri U, Njoroge SK, Witek MA, Adebiyi MG, Kamande JW, Hupert ML, Barany F, Soper SA (2011) High-throughput selection, enumeration, electrokinetic manipulation, and molecular profiling of low-abundance circulating tumor cells using a microfluidic system. Anal Chem 83(6):2301–2309. doi:10.1021/ac103172y
Farace F, Massard C, Vimond N, Drusch F, Jacques N, Billiot F, Laplanche A, Chauchereau A, Lacroix L, Planchard D, Le Moulec S, Andre F, Fizazi K, Soria JC, Vielh P (2011) A direct comparison of Cell Search and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Brit J Cancer 105(6):847–853. doi:10.1038/bjc.2011.294
Hou J, Krebs MG, Ward T, Morris K, Sloane R, Blackhall FH, Dive C (2010) Circulating tumor cells. Enumeration and beyond. Cancers 2(2):1236–1250. doi:10.3390/cancers2021236
Hupert ML, Guy WJ, Llopis SD, Shadpour H, Rani S, Nikitopoulos DE, Soper SA (2007) Evaluation of micromilled metal mold masters for the replication of microchip electrophoresis devices. Microfluid Nanofluid 3(1):1–11
Jackson JM, Witek MA, Hupert M, Brady C, Pullagurla S, Kamande J, Aufforth R, Tignanelli CJ, Torphy RJ, Yeh JJ, Soper SA (2014) UV activation of polymeric high aspect ratio microstructures: ramifications in antibody surface loading for circulating tumor cell selection. Lab Chip [Advance Article]. doi:10.1039/C3LC50618E
Kamande JW, Hupert ML, Witek MA, Wang H, Trophy R, Dharmasiri U, Njoroge SK, Jackson JM, Aufforth R, Snavely A, Yeh JJ, Soper SA (2013) Modular microsystem for the isolation, enumeration, and phenotyping of circulating tumor cells: managing patients with pancreatic cancer. Anal Chem. doi:10.1021/ac401720k
Kotz KT, Xiao W, Miller-Graziano C, Qian W-J, Russom A, Warner EA, Moldawer LL, De A, Bankey PE, Petritis BO, Camp DG, Rosenbach AE, Goverman J, Fagan SP, Brownstein BH, Irimia D, Xu W, Wilhelmy J, Mindrinos MN, Smith RD, Davis RW, Tompkins RG, Toner M (2010) Clinical microfluidics for neutrophil genomics and proteomics. Nat Med 16(9):1042–1047
Kuo JS, Zhao Y, Schiro PG, Ng L, Lim DSW, Shelby JP, Chiu DT (2010) Deformability considerations in filtraction of biological cells. Lab Chip 10:837–842
Lin YG, Merritt WM, Spannuth WA, Nick AM, Stone RL, Tsinberg P, Coleman RL, Birrer MJ, Bischoff F, Sood AK (2009) Rare circulating tumor cells can be reliably and efficiently detected using a novel microfluidics and micro-electromechanical systems (MEMS)-based rare cell recovery platform: preclinical and clinical data. Gynecol Oncol 112(2):230
Maheswaran S, Haber DA (2010) Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev 20(1):96–99. doi:10.1016/j.gde.2009.12.002
Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Lafrate AJ, Bell DW, Digumarthy S, Muzikansky A, Irimia D, Settleman J, Tompkins RG, Lynch TJ, Toner M, Haber DA (2008) Detection of mutations in EGFR in circulating lung-cancer cells. New Engl J Med 359:366–377
McCarley RL, Vaidya B, Wei S, Smith AF, Patel AB, Feng J, Murphy MC, Soper SA (2005) Resist-free patterning of surface architectures in polymer-based microanalytical devices. J Am Chem Soc 127:842–843. doi:10.1021/ja0454135
Mostert B, Kraan J, de Vries JB, van der Spoel P, Sieuwerts AM, Schutte M, Timmermans AM, Foekens R, Martens JWM, Gratama J-W, Foekens JA, Sleijfer S (2011) Detection of circulating tumor cells in breast cancer may improve through enrichment with CD-146. Breast Cancer Res Tr 127:33–41
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450(7173):1235–1239. doi:10.1038/nature06385
Nakagawa T, Martinez SR, Goto Y, Koyanagi K, Kitago M, Shingai T, Elashoff DA, Ye X, Singer FR, Giuliano AE, Hoon DS (2007) Detection of circulating tumor cells in early-stage breast cancer metastasis to axillary lymph nodes. Clin Cancer Res 13(14):4105–4110. doi:10.1158/1078-0432.CCR-07-0419
Saliba A-E, Saias L, Psychari E, Minc N, Simon D, Bidard F-C, Mathiot C, Pierga J-Y, Fraisier V, Salamero J, Saada V, Farace F, Vielh P, Malaquin L, Viovy J-L (2010) Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. P Natl Acad Sci 107(33):14524–14529. doi:10.1073/pnas.1001515107
Sieuwerts AM, Kraan J, Bolt J, van der Spoel P, Elstrodt F, Schutte M, Martens JWM, Gratama JW, Sleijfer S, Foekens JA (2009) Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Nat Cancer I 101:61–66
Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG, Connelly MC, Terstappen LW, O’Hara SM (2005) Global gene expression profiling of circulating tumor cells. Cancer Res 65(12):4993–4997. doi:10.1158/0008-5472.CAN-04-4330
Soper SA, Ford SM, Qi S, McCarley RL, Kelly K, Murphy MC (2000) Polymeric microelectromechanical systems. Anal Chem 72(19):643A–651A
Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu C-L, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S (2010) Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med 2(25):25ra23. doi:10.1126/scitranslmed.3000403
Tan SJ, Yobas L, Lee GYH, Ong CN, Lim CT (2009) Microdevice for the isolation and enumeration of cancer cells from blood. Biomed Microdev 11(4):883–892. doi:10.1007/s10544-009-9305-9
Wang S, Liu K, Liu J, Yu ZTF, Xu X, Zhao L, Lee T, Lee EK, Reiss J, Lee Y-K, Chung LWK, Huang J, Rettig M, Seligson D, Duraiswamy KN, Shen CKF, Tseng H-R (2011) Highly efficient capture of circulating tumor cells by using nanostructured silicon substrates with integrated chaotic micromixers. Angew Chem Int Edit 50(13):3084–3088. doi:10.1002/anie.201005853
Williams A, Balic M, Datar R, Cote R (2012) Size-based enrichment technologies for CTC detection and characterization. In: Ignatiadis M, Sotiriou C, Pantel K (eds) Minimal residual disease and circulating tumor cells in breast cancer, vol 195. Rec Res Cancer. Springer Berlin Heidelberg, pp 87-95. doi:10.1007/978-3-642-28160-0_8
Xu Y, Phillips JA, Yan JL, Li QG, Fan ZH, Tan WH (2009) Aptamer-based microfluidic device for enrichment, sorting, and detection of multiple cancer Cells. Anal Chem 81(17):7436–7442
Yang L, Lang JC, Balasubramanian P, Jatana KR, Schuller D, Agrawal A, Zborowski M, Chalmers JJ (2009) Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnol Bioeng 102(2):521–534. doi:10.1002/bit.22066
Yuhua G, Liu G, Xiong Y, Jun W, Xinlong H, Tian Y (2007) Study of hot embossing using nickel and Ni-PTFE LiGA mold inserts. J Microelectromech Syst 16(3):589–597. doi:10.1109/jmems.2007.896715
Zhang W, Hu P, Lai X, Peng L (2009) Analysis and optimization of flow distribution in parallel-channel configurations for proton exchange membrane fuel cells. J Power Sources 194(2):931–940. doi:10.1016/j.jpowsour.2009.05.033
Acknowledgments
The authors would like to thank the NIH (R01-EB010087; N43CO-2010-00066 (NCI-SBIR)), the University of North Carolina (UNC), the University Cancer Research Fund (UNC) and the World Class University Program in Korea for financial support of this work. The authors would also like to thank the UNC Olympus Imaging Research Center for providing the use of the microscope for CTC imaging, UNC-Chapel Hill Analytical and Nanofabrication Laboratory (UNC-CHANL) for access to hot embossing machine and SEM facilities, and North Carolina State University Precision Instrument Machine Shop for fabrication of molding masters.
Conflict of interest
The authors declare the following competing financial interest(s): M.L.H. and S.A.S. have financial interests in BioFluidica, Inc.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hupert, M.L., Jackson, J.M., Wang, H. et al. Arrays of high-aspect ratio microchannels for high-throughput isolation of circulating tumor cells (CTCs). Microsyst Technol 20, 1815–1825 (2014). https://doi.org/10.1007/s00542-013-1941-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-013-1941-6