Abstract
Purpose
The effect of anesthetic types on postoperative acute kidney injury (AKI) remains unclear particularly in patients undergoing non-cardiac surgery. The purpose of this retrospective study was to compare total intravenous anesthesia (TIVA) and inhalation anesthesia in terms of the risk of AKI after open major abdominal surgery (MAS).
Methods
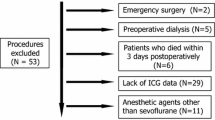
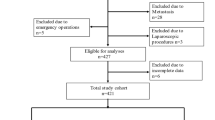
Adult patients who underwent open MAS (gastrectomy, hepatectomy, colectomy, or pancreatectomy) at our institute from 2016 to 2018 were included. Using the multivariable logistic regression, the risk of postoperative AKI was compared among patients who underwent TIVA (TIVA group) and inhalation anesthesia (inhalation group) both in the total cohort and in the propensity score-matched cohort. Additional multivariable logistic regression analysis was performed with inverse probability of treatment weighting (IPTW) using the propensity score.
Results
In total, 3616 patients were analyzed. The incidence of postoperative AKI was 5.0% (77/1546) and 7.8% (161/2070) in the TIVA and inhalation groups, respectively. The risk of AKI was significantly higher in the inhalation group [adjusted odds ratio (aOR) 1.72; 95% confidence interval (CI) 1.27–2.35; P = 0.002] than the TIVA group. In the matched cohort (n = 1518 in each group), the inhalation group also had a higher risk of AKI (aOR 1.66; 95% CI 1.20–2.31; P = 0.002). The multivariable logistic regression with IPTW showed similar results (aOR 1.59; 95% CI 1.30–1.95; P < 0.001).
Conclusions
The risk of AKI after open MAS differed significantly according to the anesthetic used. Patients receiving inhalation anesthesia may have a greater risk of postoperative AKI than those undergoing TIVA.


Similar content being viewed by others
References
Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, Hunn BH. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28:377–87.
Teixeira C, Rosa R, Rodrigues N, Mendes I, Peixoto L, Dias S, Melo MJ, Pereira M, Bicha Castelo H, Lopes JA. Acute kidney injury after major abdominal surgery: a retrospective cohort analysis. Crit Care Res Pract. 2014;2014:132175.
Tomozawa A, Ishikawa S, Shiota N, Cholvisudhi P, Makita K. Perioperative risk factors for acute kidney injury after liver resection surgery: an historical cohort study. Can J Anaesth. 2015;62:753–61.
Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–8.
Gameiro J, Fonseca JA, Neves M, Jorge S, Lopes JA. Acute kidney injury in major abdominal surgery: incidence, risk factors, pathogenesis and outcomes. Ann Intensive Care. 2018;8:22.
Kim CS, Oak CY, Kim HY, Kang YU, Choi JS, Bae EH, Ma SK, Kweon SS, Kim SW. Incidence, predictive factors, and clinical outcomes of acute kidney injury after gastric surgery for gastric cancer. PLoS ONE. 2013;8:e82289.
Kashy BK, Podolyak A, Makarova N, Dalton JE, Sessler DI, Kurz A. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology. 2014;121:730–9.
O’Connor ME, Kirwan CJ, Pearse RM, Prowle JR. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med. 2016;42:521–30.
Billings FT 4th, Ball SK, Roberts LJ 2nd, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;50:1480–7.
Billings FT 4th, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, Brown NJ. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. 2012;23:1221–8.
Runzer TD, Ansley DM, Godin DV, Chambers GK. Tissue antioxidant capacity during anesthesia: propofol enhances in vivo red cell and tissue antioxidant capacity in a rat model. Anesth Analg. 2002;94:89–93.
Motayagheni N, Phan S, Eshraghi C, Nozari A, Atala A. A review of anesthetic effects on renal function: potential organ protection. Am J Nephrol. 2017;46:380–9.
Lee HT, Chen SW, Doetschman TC, Deng C, D’Agati VD, Kim M. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol. 2008;295:F128–36.
Kong HY, Zhu SM, Wang LQ, He Y, Xie HY, Zheng SS. Sevoflurane protects against acute kidney injury in a small-size liver transplantation model. Am J Nephrol. 2010;32:347–55.
Bang JY, Lee J, Oh J, Song JG, Hwang GS. The influence of propofol and sevoflurane on acute kidney injury after colorectal surgery: a retrospective cohort study. Anesth Analg. 2016;123:363–70.
Yoo YC, Shim JK, Song Y, Yang SY, Kwak YL. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014;86:414–22.
Li H, Weng Y, Yuan S, Liu W, Yu H, Yu W. Effect of sevoflurane and propofol on acute kidney injury in pediatric living donor liver transplantation. Ann Transl Med. 2019;7:340.
Oh TK, Kim J, Han S, Kim K, Jheon S, Ji E. Effect of sevoflurane-based or propofol-based anaesthesia on the incidence of postoperative acute kidney injury: a retrospective propensity score-matched analysis. Eur J Anaesthesiol. 2019;36:649–55.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107.
Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–7.
Halpern EF. Behind the numbers: inverse probability weighting. Radiology. 2014;271:625–8.
Schulte PJ, Mascha EJ. Propensity score methods: theory and practice for anesthesia research. Anesth Analg. 2018;127:1074–84.
Chen Y, Jiang S, Wu Y. Effect of 2 different anesthesia methods on stress response in neurosurgical patients with hypertension or normal: a prospective clinical trial. Medicine (Baltimore). 2016;95:e4769.
Nadler JW, Quinn TD, Bennett-Guerrero E. Can anesthesiologists affect cancer outcomes? Can J Anaesth. 2019;66:491–4.
Jun IJ, Jo JY, Kim JI, Chin JH, Kim WJ, Kim HR, Lee EH, Choi IC. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep. 2017;7:14020.
Soltanizadeh S, Degett TH, Gogenur I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: a systematic review. J Clin Anesth. 2017;42:19–25.
Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124:69–79.
Yoo S, Lee HB, Han W, Noh DY, Park SK, Kim WH, Kim JT. Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: a retrospective cohort study. Anesthesiology. 2019;130:31–40.
Bihorac A. Acute kidney injury in the surgical patient: recognition and attribution. Nephron. 2015;131:118–22.
Birnie K, Verheyden V, Pagano D, Bhabra M, Tilling K, Sterne JA, Murphy GJ. Predictive models for kidney disease: improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care. 2014;18:606.
Echarri G, Duque-Sosa P, Callejas R, Garcia-Fernandez N, Nunez-Cordoba JM, Iribarren MJ, Monedero P, The Renal Dysfunction in Cardiac Surgery Spanish Group (GEDRCC2). External validation of predictive models for acute kidney injury following cardiac surgery: a prospective multicentre cohort study external validation of predictive models for acute kidney injury following cardiac surgery: a prospective multicentre cohort study. Eur J Anaesthesiol. 2017;34:81–8.
Englberger L, Suri RM, Li Z, Dearani JA, Park SJ, Sundt TM 3rd, Schaff HV. Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. Am J Kidney Dis. 2010;56:623–31.
Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–16.
Ng SY, Sanagou M, Wolfe R, Cochrane A, Smith JA, Reid CM. Prediction of acute kidney injury within 30 days of cardiac surgery. J Thorac Cardiovasc Surg. 2014;147:1875–83.
Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–8.
Sanchez-Conde P, Rodriguez-Lopez JM, Nicolas JL, Lozano FS, Garcia-Criado FJ, Cascajo C, Gonzalez-Sarmiento R, Muriel C. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg. 2008;106:371–8.
Rodriguez-Lopez JM, Sanchez-Conde P, Lozano FS, Nicolas JL, Garcia-Criado FJ, Cascajo C, Muriel C. Laboratory investigation: effects of propofol on the systemic inflammatory response during aortic surgery. Can J Anaesth. 2006;53:701–10.
Li Y, Zhong D, Lei L, Jia Y, Zhou H, Yang B. Propofol prevents renal ischemia-reperfusion injury via inhibiting the oxidative stress pathways. Cell Physiol Biochem. 2015;37:14–26.
Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313–24.
Acknowledgements
We specially thank Younghae Cho, B.S. (from the Department of Statistics, Sungkyunkwan University, Seoul, Korea) for his significant contribution to the statistical analyses in this work. This study used clinical data retrieved from Seoul National University Hospital Patients Research Environment (SUPREME) system.
Author information
Authors and Affiliations
Contributions
BRK, J-HB, and KN: Conceptualization; Formal analysis and investigation: BRK, SY, GYS, SL, and KN; Writing—original draft preparation: BRK, J-HB, and KN; Writing—review and editing: SY, GYS, and SL; Supervision: J-HB.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kim, B.R., Yoon, S., Song, G.Y. et al. The impact of total intravenous anesthesia versus inhalation anesthesia on acute kidney injury after major abdominal surgery: a propensity score analysis. J Anesth 35, 112–121 (2021). https://doi.org/10.1007/s00540-020-02882-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-020-02882-9