Abstract
Purpose
Previous in vitro studies have shown that degradation of opioid peptides during incubation with cerebral membrane preparations is almost completely prevented by a mixture of three peptidase inhibitors (PIs), namely, amastatin, captopril, and phosphoramidon. In the present in vivo study, we evaluate the effects of intrathecal administration of these PIs on antinociception by [Met5]enkephalin (ME) or PIs themselves.
Methods
Drugs were administered into the thoracolumbar level of the spinal cord in the intrathecal space in rat. Induction of antinociception was measured by the tail immersion assay, with 55 °C as the nociceptive stimulus. Effects of PIs on antinociception were evaluated by dose–response study (ME, 1–20 nmol; PIs, 1–20 nmol each), by comparison of differences among two combinations of PIs (amastatin and captopril; captopril and phosphoramidon; amastatin and phosphoramidon) and three PIs (amastatin, captopril, and phosphoramidon), and by using opioid receptor selective antagonists.
Results
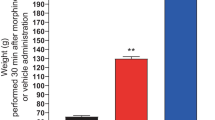
Intrathecal administration of ME with these three PIs or PIs alone significantly and dose dependently increased antinociception in a μ- and δ-opioid receptor antagonist-reversible manner; moreover, the degree of antinociception with a combination of any two of these was less than that with all three, indicating that any residual single peptidase could inactivate significant amounts of ME.
Conclusion
The present data, together with those of earlier studies, clearly demonstrate that amastatin-, captopril-, and phosphoramidon-sensitive enzymes play an important role in inactivation of opioid peptides at the spinal level.





Similar content being viewed by others
References
Morales-Mulia M, de Gortari P, Amaya MI, Mendez M. Activity and expression of enkephalinase and aminopeptidase N in regions of the mesocorticolimbic system are selectively modified by acute ethanol administration. J Mol Neurosci. 2012;46:58–67.
Khaket TP, Singh J, Attri P, Dhanda S. Enkephalin degrading enzymes: metalloproteases with high potential for drug development. Curr Pharm Des. 2012;18:220–30.
Hiranuma T, Oka T. Effects of peptidase inhibitors on the [Met5]-enkephalin hydrolysis in ileal and striatal preparations of guinea-pig: almost complete protection of degradation by the combination of amastatin, captopril and thiorphan. Jpn J Pharmacol. 1986;41:437–46.
Aoki K, Kajiwara M, Oka T. The role of bestatin-sensitive aminopeptidase, angiotensin converting enzyme and thiorphan-sensitive “enkephalinase” in the potency of enkephalins in the guinea-pig ileum. Jpn J Pharmacol. 1984;36:59–65.
Aoki K, Kajiwara M, Oka T. The inactivation of [Met5]-enkephalin by bestatin-sensitive aminopeptidase, captopril-sensitive peptidyl dipeptidase A and thiorphan-sensitive endopeptidase-24.11 in mouse vas deferens. Jpn J Pharmacol. 1986;40:297–302.
Cui SY, Kajiwara M, Ishii K, Aoki K, Sakamoto J, Matsumiya T, Oka T. The enhancing effects of amastatin, phosphoramidon and captopril on the potency of [Met5]-enkephalin in rat vas deferens. Jpn J Pharmacol. 1986;42:43–9.
Hiranuma T, Kitamura K, Taniguchi T, Kanai M, Arai Y, Iwao K, Oka T. Protection against dynorphin-(1-8) hydrolysis in membrane preparations by the combination of amastatin, captopril and phosphoramidon. J Pharmacol Exp Ther. 1998;286:863–9.
Hiranuma T, Kitamura K, Taniguchi T, Kobayashi T, Tamaki R, Kanai M, Akahori K, Iwao K, Oka T. Effects of three peptidase inhibitors, amastatin, captopril and phosphoramidon, on the hydrolysis of [Met5]-enkephalin-Arg6-Phe7 and other opioid peptides. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:276–82.
Oka T, Aoki K, Kajiwara M, Ishii K, Kuno Y, Hiranuma T, Matsumiya T. Inactivation of [Leu5]-enkephalin in three isolated preparations: relative importance of aminopeptidase, endopeptidase-24.11 and peptidyl dipeptidase A. NIDA Res Monogr. 1986;75:259–62.
Costentin J, Vlaiculescu A, Chaillet P, Ben Natan L, Aveaux D, Schwartz JC. Dissociated effects of inhibitors of enkephalin-metabolising peptidases or naloxone on various nociceptive responses. Eur J Pharmacol. 1986;123:37–44.
Norman JA, Autry WL, Barbaz BS. Angiotensin-converting enzyme inhibitors potentiate the analgesic activity of [Met]-enkephalin-Arg6-Phe7 by inhibiting its degradation in mouse brain. Mol Pharmacol. 1985;28:521–6.
Stine SM, Yang HY, Costa E. Inhibition of in situ metabolism of [3H](Met5)-enkephalin and potentiation of (Met5)-enkephalin analgesia by captopril. Brain Res. 1980;188:295–9.
Van Amsterdam JG, Llorens-Cortes C. Inhibition of enkephalin degradation by phelorphan: effects on striatal [Met5]enkephalin levels and jump latency in mouse hot plate test. Eur J Pharmacol. 1988;154:319–24.
Kuno Y, Aoki K, Kajiwara M, Ishii K, Oka T. The relative potency of enkephalins and beta-endorphin in guinea-pig ileum, mouse vas deferens and rat vas deferens after the administration of peptidase inhibitors. Jpn J Pharmacol. 1986;41:273–81.
Oka T, Liu XF, Kajita T, Ohgiya N, Ghoda K, Taniguchi T, Arai Y, Matsumiya T. Effects of the subcutaneous administration of enkephalins on tail-flick response and righting reflex of developing rats. Brain Res Dev Brain Res. 1992;69:271–6.
Akahori K, Kosaka K, Jin XL, Arai Y, Yoshikawa M, Kobayashi H, Oka T. Great increase in antinociceptive potency of [Leu5]enkephalin after peptidase inhibition. J Pharmacol Sci. 2008;106:295–300.
Kitamura K, Akahori K, Yano H, Iwao K, Oka T. Effects of peptidase inhibitors on anti-nociceptive action of dynorphin-(1-8) in rats. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:273–8.
Walker EA. In vivo pharmacological resultant analysis reveals noncompetitive interactions between opioid antagonists in the rat tail-withdrawal assay. Br J Pharmacol. 2006;149:1071–82.
Xie H, Woods JH, Traynor JR, Ko MC. The spinal antinociceptive effects of endomorphins in rats: behavioral and G protein functional studies. Anesth Analg. 2008;106:1873–81.
Malmberg AB, Yaksh TL. Isobolographic and dose–response analyses of the interaction between intrathecal mu and delta agonists: effects of naltrindole and its benzofuran analog (NTB). J Pharmacol Exp Ther. 1992;263:264–75.
Barnes K, Turner AJ. The endothelin system and endothelin-converting enzyme in the brain: molecular and cellular studies. Neurochem Res. 1997;22:1033–40.
Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–6.
Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawal reflex in rats. Arzneimittelforschung. 1963;13:502–7.
Kishioka S, Miyamoto Y, Fukunaga Y, Nishida S, Yamamoto H. Effects of a mixture of peptidase inhibitors (amastatin, captopril and phosphoramidon) on Met-enkephalin-, beta-endorphin-, dynorphin-(1-13)- and electroacupuncture-induced antinociception in rats. Jpn J Pharmacol. 1994;66:337–45.
Taniguchi T, Fan XT, Kitamura K, Oka T. Effects of peptidase inhibitors on the enkephalin-induced anti-nociception in rats. Jpn J Pharmacol. 1998;78:487–92.
Noble F, Soleilhac JM, Soroca-Lucas E, Turcaud S, Fournie-Zaluski MC, Roques BP. Inhibition of the enkephalin-metabolizing enzymes by the first systemically active mixed inhibitor prodrug RB 101 induces potent analgesic responses in mice and rats. J Pharmacol Exp Ther. 1992;261:181–90.
Chen W, Song B, Lao L, Perez OA, Kim W, Marvizon JC. Comparing analgesia and mu-opioid receptor internalization produced by intrathecal enkephalin: requirement for peptidase inhibition. Neuropharmacology. 2007;53:664–76.
Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–50.
Lu Y, Sweitzer SM, Laurito CE and Yeomans DC. Differential opioid inhibition of C- and A delta- fiber mediated thermonociception after stimulation of the nucleus raphe magnus. Anesth Analg 2004;98:414–419, table of contents
Przewlocka B, Mika J, Labuz D, Toth G, Przewlocki R. Spinal analgesic action of endomorphins in acute, inflammatory and neuropathic pain in rats. Eur J Pharmacol. 1999;367:189–96.
Noble F, Banisadr G, Jardinaud F, Popovici T, Lai-Kuen R, Chen H, Bischoff L, Parsadaniantz SM, Fournie-Zaluski MC, Roques BP. First discrete autoradiographic distribution of aminopeptidase N in various structures of rat brain and spinal cord using the selective iodinated inhibitor [125I]RB 129. Neuroscience. 2001;105:479–88.
Waksman G, Hamel E, Fournie-Zaluski MC, Roques BP. Autoradiographic comparison of the distribution of the neutral endopeptidase “enkephalinase” and of mu and delta opioid receptors in rat brain. Proc Natl Acad Sci USA. 1986;83:1523–7.
Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38.
Blum K, Briggs AH, Trachtenberg MC, Delallo L, Wallace JE. Enkephalinase inhibition: regulation of ethanol intake in genetically predisposed mice. Alcohol. 1987;4:449–56.
Kanai M, Takahashi S, Kosaka K, Iwao K, Kobayashi H, Oka T. [Met5]Enkephalin-Arg-Gly-Leu-induced antinociception is greatly increased by peptidase inhibitors. Eur J Pharmacol. 2002;453:53–8.
Miura M, Yoshikawa M, Watanabe M, Takahashi S, Ajimi J, Ito K, Ito M, Kawaguchi M, Kobayashi H, Suzuki T. Increase in antinociceptive effect of [leu5]enkephalin after intrathecal administration of mixture of three peptidase inhibitors. Tokai J Exp Clin Med. 2013;38:62–70.
Simantov R, Snyder SH. Morphine-like peptides, leucine enkephalin and methionine enkephalin: interactions with the opiate receptor. Mol Pharmacol. 1976;12:987–98.
Schwartz JC, Malfroy B, De La Baume S. Biological inactivation of enkephalins and the role of enkephalin-dipeptidyl-carboxypeptidase (“enkephalinase”) as neuropeptidase. Life Sci. 1981;29:1715–40.
Corbett AD, Henderson G, McKnight AT, Paterson SJ. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol. 2006;147(suppl 1):S153–62.
Jutkiewicz EM. RB101-mediated protection of endogenous opioids: potential therapeutic utility? CNS Drug Rev. 2007;13:192–205.
Thanawala V, Kadam VJ, Ghosh R. Enkephalinase inhibitors: potential agents for the management of pain. Curr Drug Targets. 2008;9:887–94.
Acknowledgments
The authors thank Professor Jeremy Williams, Tokyo Medical University, for his assistance with the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Murata, T., Yoshikawa, M., Watanabe, M. et al. Potentiation of [Met5]enkephalin-induced antinociception by mixture of three peptidase inhibitors in rat. J Anesth 28, 708–715 (2014). https://doi.org/10.1007/s00540-014-1819-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-014-1819-5