Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) are characterized by the accumulation of excess hepatic fat. However, in the progression from NASH to cirrhosis, hepatic fat is often lost. Our aim was to elucidate the mechanism underlying hepatic fat loss during NASH progression.
Methods
Liver biopsies were performed at The University of Tokyo Hospital between November 2011 and March 2016 on 146 patients with NAFLD and 14 patients with cryptogenic cirrhosis who were not being treated with any diabetes or dyslipidemia drugs. Among them, 70 patients underwent liver biopsy after an overnight fast, and 90 patients were biopsied 5 h after an oral glucose tolerance test. Expression differences in genes encoding several fatty acid metabolism-related factors were examined and correlated with hepatic histological changes based on NAFLD activity scores. Prospective patient follow-up continued until June 2018.
Results
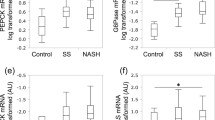
The level of fatty acid transport protein 5 (FATP5), which is associated with free fatty acid intake, was significantly and inversely correlated with features of histological progression, including ballooning and fibrosis. This was confirmed by immunohistochemical analysis. Transcript levels of genes encoding fatty acid metabolism-related proteins were comparable between NASH with severe fibrosis and cryptogenic cirrhosis. Furthermore, a prospective cohort study demonstrated that low FATP5 expression was the most significant risk factor for hepatic fat loss.
Conclusions
Decreased hepatic FATP5 expression in NAFLD is linked to histological progression, and may be associated with hepatic fat loss during NASH progression to cirrhosis.






Similar content being viewed by others
References
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Caldwell S, Marchesini G. Cryptogenic vs. NASH-cirrhosis: the rose exists well before its name. J Hepatol. 2018;68:391–2.
Caldwell SH, Lee VD, Kleiner DE, et al. NASH and cryptogenic cirrhosis: a histological analysis. Ann Hepatol. 2009;8:346–52.
Mulhall BP, Ong JP, Younossi ZM. Non-alcoholic fatty liver disease: an overview. J Gastroenterol Hepatol. 2002;17:1136–43.
Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Investig. 2005;115:1343–51.
Wilding JP. The importance of free fatty acids in the development of type 2 diabetes. Diabet Med. 2007;24:934–45.
Gambino R, Bugianesi E, Rosso C, et al. Different serum free fatty acid profiles in NAFLD subjects and healthy controls after oral fat load. Int J Mol Sci. 2016;17:479.
Zhang J, Zhao Y, Xu C, et al. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4:5832.
Bradbury MW. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194–98.
Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–89.
Schneider H, Staudacher S, Poppelreuther M, et al. Protein mediated fatty acid uptake: synergy between CD36/FAT-facilitated transport and acyl-CoA synthetase-driven metabolism. Arch Biochem Biophys. 2014;546:8–18.
Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–85.
Schwarz JM, Linfoot P, Dare D, et al. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77:43–50.
Kotronen A, Seppälä-Lindroos A, Vehkavaara S, et al. Liver fat and lipid oxidation in humans. Liver Int. 2009;29:1439–46.
Miele L, Grieco A, Armuzzi A, et al. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol. 2003;98:2335–6.
Nakamuta M, Kohjima M, Higuchi N, et al. The significance of differences in fatty acid metabolism between obese and non-obese patients with non-alcoholic fatty liver disease. Int J Mol Med. 2008;22:663–7.
Dasarathy S, Yang Y, McCullough AJ, et al. Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2011;23:382–8.
Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Okushin K, Tsutsumi T, Enooku K, et al. The intrahepatic expression levels of bile acid transporters are inversely correlated with the histological progression of nonalcoholic fatty liver disease. J Gastroenterol. 2016;51:808–18.
Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–50.
Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–68.
Myers RP, Pollett A, Kirsch R, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–10.
Lee HW, Park SY, Kim SU, et al. Discrimination of nonalcoholic steatohepatitis using transient elastography in patients with nonalcoholic fatty liver disease. PLoS One. 2016;11:e0157358.
Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30.
Jungermann K. Metabolic zonation of liver parenchyma. Semin Liver Dis. 1988;8:329–41.
Enooku K, Kondo M, Fujiwara N, et al. Hepatic IRS1 and β-catenin expression is associated with histological progression and overt diabetes emergence in NAFLD patients. J Gastroenterol. 2018;53:1261–75.
Männistö VT, Simonen M, Hyysalo J, et al. Ketone body production is differentially altered in steatosis and non-alcoholic steatohepatitis in obese humans. Liver Int. 2015;35:1853–61.
Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio metabolism study. Diabetes. 2017;66:815–22.
Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res. 1995;36:229–40.
Stahl A, Gimeno RE, Tartaglia LA, et al. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol Metab. 2001;12:266–73.
Steneberg P, Sykaras AG, Backlund F, et al. Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes hepatosteatosis and hepatic insulin resistance. J Biol Chem. 2015;290:19034–43.
Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–67.
Garbacz WG, Lu P, Miller TM, et al. Hepatic overexpression of CD36 improves glycogen homeostasis and attenuates high-fat diet-induced hepatic steatosis and insulin resistance. Mol Cell Biol. 2016;36:2715–27.
Martin GG, Danneberg H, Kumar LS, et al. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J Biol Chem. 2003;278:21429–38.
Doege H, Grimm D, Falcon A, et al. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008;283:22186–92.
Zhu L, Baker SS, Liu W, et al. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: combined effects of pathways on steatosis. Metabolism. 2011;60:1001–11.
Tsai JH, Ferrell LD, Tan V, et al. Aggressive non-alcoholic steatohepatitis following rapid weight loss and/or malnutrition. Mod Pathol. 2017;30:834–42.
Shaffer EA. Bariatric surgery: a promising solution for nonalcoholic steatohepatitis in the very obese. J Clin Gastroenterol. 2006;40(Suppl 1):S44–S50.
Mattar SG, Velcu LM, Rabinovitz M, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610–7 (discussion 8–20).
Müller MJ, Rieger A, Willmann O, et al. Metabolic responses to lipid infusions in patients with liver cirrhosis. Clin Nutr. 1992;11:193–206.
Richardson RA, Davidson HI, Hinds A, et al. Influence of the metabolic sequelae of liver cirrhosis on nutritional intake. Am J Clin Nutr. 1999;69:331–7.
Horton JD, Bashmakov Y, Shimomura I, et al. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA. 1998;95:5987–92.
Acknowledgements
We thank Ms. Seiko Shinzawa for technical assistance. This work was supported by the Research Program on Hepatitis from the Japan Agency for Medical Research and Development (JP17fk0210304 and JP18fk0210040), and from the Ministry of Education, Culture, Sports, Science and Technology. No additional external funding was received. The funders played no role in the study design, data collection, analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2019_1633_MOESM2_ESM.pdf
FATP5 and CPT1A mRNA expression in patients with autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC) In patients with AIH and PBC, FATP5 and CPT1A levels did not show significant decreases compared to patients designated as Matteoni type 1 or 2. (PDF 792 kb)
535_2019_1633_MOESM3_ESM.pdf
Binary images of FATP5 immunostaining in patients A-F. These binary images were obtained from images in Figure 3. (PDF 1390 kb)
535_2019_1633_MOESM4_ESM.pdf
Binary images of CPT1A immunostaining in patients A-F These binary images were obtained from images in Figure 4. (PDF 1541 kb)
535_2019_1633_MOESM5_ESM.pdf
FATP5 and CPT1A expression in binary images We constructed binary images of FATP5 and CPT1A immunostaining in five patients with severe ballooning, five patients without ballooning, and five patients with cirrhosis in both the fasting and glucose-loaded groups. The percentages of the FATP5 and CPT1A reactive areas were measured compared to the total area at 200× magnification. Two fields showing the periportal regions of each patient were successively imaged for histomorphometric evaluation. Mann–Whitney U tests were used to investigate differences, and two-tailed P values < 0.05 were considered statistically significant. (PDF 809 kb)
535_2019_1633_MOESM6_ESM.pdf
Cumulative incidence of hepatic steatosis loss (controlled attenuation parameter [CAP] < 250dB/m) without weight reduction stratified by the FATP5/GAPDH ratio in patients with CAP > 250dB/m at liver biopsy The median follow-up period was 3.69 years (IQR 3.34–4.26 years). (PDF 114 kb)
Rights and permissions
About this article
Cite this article
Enooku, K., Tsutsumi, T., Kondo, M. et al. Hepatic FATP5 expression is associated with histological progression and loss of hepatic fat in NAFLD patients. J Gastroenterol 55, 227–243 (2020). https://doi.org/10.1007/s00535-019-01633-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01633-2