Abstract
Background
Novel risk factors for lymph node metastasis (LNM) in T1 colorectal cancer (CRC) have been recently proposed, but most have not been implemented because of the lack of validation. Here we determined the value of poorly differentiated clusters (PDCs) in a multi-institutional cohort of T1 CRC cases.
Methods
A pathology review involving 30 institutions was conducted for 3556 T1 CRCs. PDC was defined as malignant clusters comprising ≥5 cells and lacking a glandular formation. The ability to identify LNM risk was compared using Akaike’s information criterion (AIC).
Results
PDC was observed in 1401 tumors (39.4 %), including 94 (17.8 %) with <1000 µm submucosal invasion and 1307 (43.2 %) with ≥1000 µm submucosal invasion (P < 0.0001). The incidence of LNM was higher in PDC-positive tumors (17.4 %) than in PDC-negative tumors (6.9 %; P < 0.0001), and PDCs had an adverse impact on LNM irrespective of the degree of submucosal invasion. Grade 3, vascular invasion, budding, and submucosal invasion depth were also significant factors (all, P < 0.0001). AIC of risk factor to identify LNM risk was most favorable for vascular invasion (2273.4), followed by PDC (2357.4); submucosal invasion depth (2429.1) was the most unfavorable. Interinstitutional judgment disparities were smaller in PDC (kappa, 0.51) than vascular invasion (0.33) or tumor grade (0.48).
Conclusions
PDC is a promising new parameter with good ability to identify LNM risk. Use of its appropriate judgment criteria will enable us determine whether an observational policy can be safely applied following local tumor excision in T1 CRC cases.
Similar content being viewed by others
Introduction
Recent progress in endoscopic resection, including the innovative technique of endoscopic submucosal dissection, has technically resulted in lesser dependence on laparotomy for patients with early invasive (T1) colorectal cancer (CRC) [1–3]. However, indicators for accurate assessment of the risk of lymph node metastasis (LNM) in patients with resected malignant polyps and identification of the parameters that should be integrated as criteria to determine requirement of additional laparotomy for lymph node dissection remain controversial.
Initial series of study to identify histopathological risk factors for LNM in early invasive CRC first appeared in the 1980s, and since then, unfavorable tumor grade [4–9] and vascular invasion [4, 6–10] have been regarded as important adverse features. According to the National Comprehensive Cancer Network (NCCN) guidelines [11], grade 3 and positive lymphovascular invasion are the only risk factors indicating the necessity of additional surgery for patients undergoing local excision of T1 CRC with a histopathologically confirmed negative margin. However, the issue of appropriate selection of patients at risk of LNM is not simply resolved by evaluating these two parameters because, in 1–5 % of T1 CRC cases, LNM reportedly occurs in tumors with neither unfavorable tumor grade nor vascular invasion [12–14]. Until the 1990s, it was justified to overlook such false-negative incidences because of the high rate of postoperative in-hospital mortality [15], which was reportedly around 7 % for curatively intended colectomies [16]. However, recent improvements in perioperative management has reduced the mortality rate associated with additional colectomy, which is now almost insignificant [2, 17, 18]. Based on this background, many studies have been conducted to attempt to identify novel parameters other than tumor grade and vascular invasion as standards for identifying patients in whom the safety of applying this observational policy will be warranted.
Consequently, new promising risk factors for CRC have been reported (Table 1) and some have already been adopted in recent guidelines as diagnostic parameters. In particular, tumor budding is listed in the guidelines of the European Society for Medical Oncology (ESMO) guidelines [19] and the Japanese Society for Cancer of the Colon and Rectum (JSCCR) [20]. Submucosal invasion depth (the 1000-µm rule) is recognized by the JSCCR guidelines [20] as a parameter indicating the necessity of additional laparotomy. Furthermore, recent studies have reported that a histological feature of poorly differentiated clusters (PDCs) reflects the metastatic potential of CRC more accurately than conventional histopathological features such as tumor grade and vascular invasion or tumor budding [21, 22]. Likewise, PDC assessment is reportedly a potential useful index of LNM risk in early invasive CRC [23].
The growing pursuit of novel indicators over the last decade has raised expectations of further advancement in the treatment of early invasive CRC. However, most proposed parameters have not been implemented because of the lack of reproducibility, validation, and/or standardization. Furthermore, few studies have compared the value of these new factors in a large multi-institutional case series of early invasive CRC. Therefore, in the present study, we compared the value of conventional and novel histopathological risk factors, including PDC, to predict the risk of LNM on the basis of Akaike’s information criterion (AIC). Our aim in this study involving 30 institutions specialized in treatment of CRC was to evaluate the potential value of new risk factors and clarify potential issues resulting from the use of these factors in routine practice.
Patients and methods
Patients
A total of 3556 early invasive CRCs were reviewed for pathology in 30 institutions belonging to the JSCCR. These institutions included university and major regional hospitals as well as cancer centers. All patients were consecutively treated in each institution within a voluntary period of time between 1980 and 2011 through laparotomy with lymph node dissection, and all tumors had their pathology confirmed as invading the submucosa. The average patient age was 64.7 years (range 23–97), and 2174 and 1382 tumors were resected from male and female patients, respectively. The median number of patients per institution enrolled in this study was 74 (range 10–478). All data except for those in interobserver study were collected from each institution by means of questionnaires in unlinkable anonymizong status. The ethics committee of the JSCCR approved the study protocol.
Evaluation of LNM parameters
In addition to the conventional parameters of tumor grade and vascular invasion, submucosal invasion depth, tumor budding, and PDC were evaluated in each institution without knowledge of the nodal status and clinical outcome to determine a potential correlation with LNM.
Submucosal invasion depth was evaluated according to the JSCCR guidelines [20]. In brief, the invasion depth was measured from the lower border of the muscularis mucosae (MM; when it was possible to identify or estimate the location of the MM) or from the surface of the polyp. For pedunculated lesions with a tangled MM (Peutz–Jeghers-type polyp), Haggitt’s level 2 [24] was used as the reference line of measurement.
Tumor budding was assessed by hematoxylin and eosin (H&E) staining and defined as an isolated single cancer cell or a cluster comprising <5 cancer cells, as reported previously [25], and after choosing a microscopic field with intense budding, a count was performed (magnification, 20× objective lens). Tumors with <5 budding foci were classified as low-grade and those with ≥5 budding foci as high-grade [20, 25].
PDC assessment
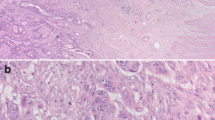
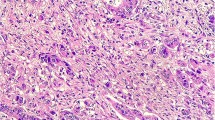
PDCs are defined as cancer clusters of ≥5 cancer cells infiltrating the stroma and lacking a glandular formation (Fig. 1) [21, 23]. H&E-stained tumor tissues were evaluated for the presence of PDCs, and positive judgment was made regardless of whether they are located inside or outside the vascular space. With regard to assessment in mucinous carcinoma, malignant clusters exhibiting the abovementioned features (i.e., stromal infiltration with minimal extracellular mucin formation) are classified as PDCs. On the other hand, cancer cell clusters within a large mucin pool (a so-called mucinous lake) are not classified as PDCs.
Poorly differentiated clusters (PDCs). a Cancer cell clusters located in the stroma, comprising ≥5 cancer cells, and lacking a glandular formation (arrows) are classified as PDCs (H&E staining; ×40 objective lens); b PDCs with intracytoplasmic lumina (ICL) or structures morphologically similar to ICL, such as cytoplasmic vacuoles and intracytoplasmic mucin (arrow head) (H&E; ×40 objective lens). a, b Extracts from the website for the multi-institution study for PDC at the 75th JSCCR meeting (http://jsccr.umin.jp/75/enq1.html)
Detailed descriptions concerning the definition and positive judgment criteria of PDCs appear on the website for this multi-institution study (http://jsccr.umin.jp/75/enq1.html). No direct instructions were provided to the observers regarding microscopic evaluations of PDCs.
Interobserver study
We conducted a study to examine the interobserver reproducibility of PDC evaluations. In brief, H&E-stained glass slides of 50 randomly selected cases of early invasive CRCs treated with endoscopic resection (R0) between 1987 and 2001 were prepared at the National Defense Medical College (NDMC). They were circulated among 10 institutions (Keiyukai Sapporo Hospital, Takano Hospital, Kurume University Faculty of Medicine, NDMC, Yamagata Prefectural Central Hospital, Hiroshima City Asa Hospital, Teikyo University School of Medicine, Aichi Cancer Center Hospital, Tokyo Women’s Medical University Medical Center East, and Osaka Medical Center for Cancer and Cardiovascular Diseases) and the diagnostic results were compared. These 10 institutions were selected in order of the number of the subjects they enrolled from 22 institutions who entered themselves for the interobserver study.
Statistical analyses
Stratification of the risk of LNM according to conventional and new risk factors was evaluated using AIC [26], which was analyzed in a logistic regression model to identify the risk factor with the highest ability to identify the risk of LNM. The optimum model (i.e., the simplest effective model with the smallest information loss when predicting outcome) will have the lowest AIC value. To assess variability of the interobserver assessments of PDCs, Cohen’s kappa statistic for all possible pairs of 10 institutions and Fleiss’ kappa statistic were calculated. For comparison, corresponding statistics for tumor grade and vascular invasion were also obtained from the same sample set.
Statistical analyses were performed using three software packages: SPSS (SPSS, Inc., Chicago, IL, USA), Stata/SE 10 (StataCorp LP, College Station, TX, USA), and R (version 2.8.1, http://www.r-project.org/) [27].
Results
Incidence of PDC
PDC was observed in 1401 tumors (39.4 %), including 94 (17.8 %) with submucosal invasion <1000 µm and 1307 (43.2 %) with ≥1000 µm submucosal invasion (P < 0.0001) (Table 2). Although there was a strong correlation between tumor grade and PDC (P < 0.0001), PDCs were observed in 32.6 % of grade 1 tumors. The incidence of PDCs was also significantly associated with vascular invasion and the grade of tumor budding (both, P < 0.0001).
Risk factors for LNM
LNM was observed in 393 tumors (11.1 %) and submucosal invasion depth, tumor grade, vascular invasion, grade of tumor budding, and PDC were all significantly associated with the incidence of LNM (Table 3). The incidence of LNM was 17.4 and 6.9 % in tumors with and without PDCs, respectively (P < 0.0001). PDCs had an adverse impact on LNM irrespective of the degree of submucosal invasion (Table 3).
Comparison of risk factors in terms of their ability to identify the risk of LNM.
AIC was used to compare the ability of all risk factors to identify the risk of LNM. As shown in Table 4, AIC was favorable in the order of vascular invasion, PDC, tumor budding, and tumor grade. Submucosal invasion depth was demonstrated to have the lowest ability to identify the risk of LNM. Odds ratio (OR) for LNM associated with grade 3 was high [OR, 7.2; 95 % confidence interval (CI), 4.4–11.8]; however, the results of AIC indicated that other risk factors (vascular invasion, budding, and PDC) had better ability to identify the possibility of LNM irrespective of the degree of submucosal invasion.
Interobserver reproducibility of PDC
Fleiss’ kappa value for risk factors among all 10 institutions was 0.51 for PDC, 0.48 for tumor grade, 0.33 for vascular invasion, 0.29 for tumor budding, and 0.21 for submucosal invasion depth.
Figure 2 shows the range of Cohen’s kappa value for all possible pairs of 10 institutions regarding PDC, tumor grade, and vascular invasion. The range was 0.33–0.80 for PDC and 0.01–0.77 for vascular invasion. None of the 50 circulated tumor specimens was diagnosed as grade 3 tumor differentiation in one institution. Thus, the range of Cohen’s kappa value for all possible pairs of the remaining nine institutions was −0.03 to 1.00 for tumor grade.
Kappa values for PDC for all possible combinations of institutional pairs. Calculated Cohen’s kappa values for all possible pairs of 10 institutions were plotted for PDC and vascular invasion in order of the values. With regard to tumor grade, none of the 50 tumor specimens circulated were diagnosed as grade 3 in one institution; thus, the kappa values for all possible pairs of the remaining nine institutions were calculated. PDCs, poorly differentiated clusters
Discussion
Over the last decade, various histopathological parameters have been proposed as potential risk factors for LNM in early invasive CRC, including those associated with the degree of submucosal invasion (the level of the polyp [24], relative level of the submucosal layer [28], measured depth [29] or width [30] of submucosal invasion, and the status of the muscularis mucosae [31]), cancer morphology (budding [32], growth pattern [33], and PDC [21]), and host response against tumor growth (inflammatory cell infiltration [34], myofibroblast proliferation [35], and microvessel count [36]). However, systematic review of articles disclose that different assessment criteria were often used for each parameter, and there have been a limited number of parameters (e.g., Haggitt’s level, relative depth of submucosal invasion, calculated depth (µm) of submucosal invasion, and tumor budding) for which positive results were validated in other studies on the basis of same assessment criteria (Table 1).
As a quantitative risk factor, the actual depth of submucosal invasion has been regarded as an important parameter of LNM in Japan [25, 30, 31]. That is because Haggitt’s classification criteria [24] poses problems for sessile-type cancers, which are always classified as level 4, and relative submucosal invasion levels defined by Kudo’s classification system [28] are difficult to apply to endoscopic resection specimens, which usually do not include the muscularis propria [37]. Based on the results of a multi-institution study to determine the cut-off point of the depth of submucosal invasion [38], the 1000-µm rule, which includes the concept of Haggitt’s level 2 as a yardstick for measurement of pedunculated polyps, has been adopted as a risk factor in the JSCCR guidelines [20]. In the present study, we found that submucosal invasion depth >1000 µm was significantly relevant to the incidence of LNM, and in particular, the false-negative rate (the incidence of positive LNM in the no-risk group) was most favorable among the parameters examined. However, it should be noted that the statistical power of identifying the risk of LNM was the lowest. Furthermore, a previous study demonstrated that approximately 80 % of malignant polyps treated with laparotomy meet the criterion of submucosal invasion >1000 µm, and this criterion was related to the LNM rate with an increase of only around 2 % compared with the average incidence of LNM in early invasive CRCs [38]. Consequently, we concluded that the 1000-µm rule has excellent power to identify cases with a very low risk of LNM; however, this criterion could increase the incidence of excessive indication of laparotomy.
Tumor budding is a histological feature of relatively small cancer foci detached from the tumor body at the invasive frontal region. The morphological features of tumor budding are put into context with the expanding knowledge of the epithelial–mesenchymal transition, which allows a polarized cell to assume a more mesenchymal phenotype with increased migratory capacity [39]. Although different assessment methods have been reported [40], budding has been recently defined in many studies as an isolated single cancer cell or a cluster of <5 cancer cells and as a significant risk factor for LNM in early invasive CRC when positive judgment was applied for ≥5 budding foci in a microscopic field of 20× objective lens [25, 31, 41–46]. This method was recently adopted by the JSCCR guidelines to evaluate tumor budding as an indicator of additional laparotomy following endoscopic treatment [20]. As expected, the present large-scale multi-institution study confirmed the adverse impact of high-grade budding on LNM on the basis of this assessment method.
More recent studies have highlighted the potential importance of PDC defined as ≥5 cancer cells with no gland formation as a new histological benchmark of CRC metastatic behavior [22, 23], and the present study confirmed the significant impact of PDC on LNM in early invasive CRC, demonstrated previously in a single-institution study [21]. The results showed that the statistical power of PDC to help in identification of the risk of LNM was the second best among the variables examined, i.e., after vascular invasion. It is generally recommended that the feature of leading edge of tumor is not included in grading tumor [47–50]. However, of note, AIC for identifying LNM risk in PDC was superior to that in tumor grade diagnosed when ignoring the finding of focal dedifferentiation at the invasive margin. Our data were consistent with the hypothesis proposed by a group of Italian surgical pathologists who stated that focally undifferentiated components, which invade fibrous tissue or the vasculature, probably play a fundamental role in lesion development [9].
With regard to judgment reproducibility, PDC was shown to be more favorable compared with conventional tumor grade [22, 23]. Our multicenter interobserver study also indicated that interinstitutional judgment disparities were smaller in PDC than that in cases of tumor grade or vascular invasion. In addition, the cut-off number of cancer cells in a cluster was arbitrarily determined for the purpose of deriving a rigorous definition for this parameter. Because PDCs do not include single cancer cells or very small clusters, which are reportedly difficult to assess in tumors with inflammatory cells using H&E-stained slides [31], improved interobserver agreements could be expected in the assessment of PDC compared with that of tumor budding, for which the requirement of cytokeratin staining for accurate identification of tumor buds has been repeatedly discussed [44, 51–55].
We believe that there is room for improvement with regard to the interobserver variation of PDC, which has not been evaluated in research trials or routine practice in any of the institutions participating in this study. Educational and training programs, as well as learning curves based on the experience gathered in routine practice expectantly make this parameter a useful tool in deciding treatment for patients with early invasive CRC.
Genetic molecular biomarkers including MSS, KRAS, or BRAF have recently been included as prognostic markers in the international guidelines [19]. With regard to the choice of treatment for early invasive CRC, however, it is unlikely that a molecular biological approach is very useful at present, and histopathological-based approaches will continue to be mainstream for a while. Compared with studies before the 1990s, in which most included <100 cases of early invasive CRC [25], we increased the number of cases analyzed within each institution (Table 1). Nevertheless, we believe a multi-institutional approach involving a large number of pathologists is essential to evaluate the value of new risk factors. In addition, the prevalence of LNM might be higher in retrospective surveys on only CRCs treated by surgery than the true prevalence because it seems possible that CRCs, which were less likely to have LMN, were excluded from the analysis. This would be likely to have contributed to a bias for the interpretation of the diagnostic accuracy for LNM. We need well-designed prospective cohort studies and on the basis of such an approach, a robust international standard for the risk assessment of locally excised early invasive CRC will be established.
Abbreviations
- LNM:
-
Lymph node metastasis
- CRC:
-
Colorectal cancer
- PDC:
-
Poorly differentiated cluster
- AIC:
-
Akaike’s information criterion
- JSCCR:
-
Japanese Society for Cancer of the Colon and Rectum
- MM:
-
Muscularis mucosa
- H&E:
-
Hematoxylin and eosin
- ICL:
-
Intracytoplasmic lumina
- OR:
-
Odds ratio
- CI:
-
Confidence interval
References
Repici A, Hassan C, Pessoa DDP, Pagano N, Arezzo A, Zullo A, et al. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy. 2012;44:137–47.
Kiriyama S, Saito Y, Yamamoto S, Soetikno R, Matsuda T, Nakajima T, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy. 2012;44:1024–30.
Lee EJ, Lee JB, Lee SH, Kim DS, Lee DH, Lee DS, et al. Endoscopic submucosal dissection for colorectal tumors-1000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc 2012 (Epub ahead of print).
Cooper HS. Surgical pathology of endoscopically removed malignant polyps of the colon and rectum. Am J Surg Pathol. 1983;7:613–23.
Morson BC, Whiteway JE, Jones EA, Macrae FA, Williams CB. Histopathology and prognosis of malignant colorectal polyps treated by endoscopic polypectomy. Gut. 1984;25:437–44.
Colacchio TA, Forde KA, Scantlebury VP. Endoscopic polypectomy: inadequate treatment for invasive colorectal carcinoma. Ann Surg. 1981;194:704–7.
Sugihara K, Muto T, Morioka Y. Management of patients with invasive carcinoma removed by colonoscopic polypectomy. Dis Colon Rectum. 1989;32:829–34.
Cranley JP, Petras RE, Carey WD, Paradis K, Sivak MV. When is endoscopic polypectomy adequate therapy for colonic polyps containing invasive carcinoma? Gastroenterology. 1986;91:419–27.
Coverlizza S, Risio M, Ferrari A, Fenoglio-Preiser CM, Rossini FP. Colorectal adenomas containing invasive carcinoma: pathologic assessment of lymph node metastatic potential. Cancer. 1989;64:1937–47.
Muller S, Chesner IM, Egan MJ, Rowlands DC, Collard MJ, Swarbrick ET, et al. Significance of venous and lymphatic invasion in malignant polyps of the colon and rectum. Gut. 1989;30:1385–91.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology-colon cancer (version 4. 2013). 2012. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed Dec 3 2012.
Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004;240:832–9.
Suh JH, Han KS, Kim BC, Hong CW, Sohn DK, Chang HJ, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy. 2012;44:590–5.
Hassan C, Zullo A, Risio M, Rossini FP, Morini S. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled-data analysis. Dis Colon Rectum. 2005;48:1588–96.
Volk EE, Goldblum JR, Petras RE, Carey WD, Fazio VW. Management and outcome of patients with invasive carcinoma arising in colorectal polyps. Gastroenterology. 1995;109:1801–7.
Fielding LP, Phillips RKS, Hittinger R. Factors influencing mortality after curative resection for large bowel cancer in elderly patients. Lancet. 1989;18:595–7.
Boenicke L, Fein M, Sailer M, Isbert C, Germer C-T, Thalheimer A. The concurrence of histologically positive resection margins and sessile morphology is an important risk factor for lymph node metastasis after complete endoscopic removal of malignant colorectal polyps. Int J Colorectal Dis. 2010;25:433–8.
Benizri EI, Bereder J-M, Rahili A, Bernard J-L, Vanbiervliet G, Filippi J, et al. Additional colectomy after colonoscopic polypectomy for T1 colon cancer: a fine balance between oncologic benefit and operative risk. Int J Colorectal Dis. 2012;27:1473–8.
Schmoll HJ, Van Cutsem V, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516.
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2009;17:1–29.
Ueno H, Hashiguchi Y, Kajiwara Y, Shinto E, Shimazaki H, Kurihara H, et al. Proposed objective criteria for “grade 3” in early invasive colorectal cancer. Am J Clin Pathol. 2010;134:312–22.
Barresi V, Bonetti LR, Branca G, Gregorio CD, de Leon MP, Tuccari G. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch. 2012;461:621–8.
Ueno H, Shimazaki H, Shinto E, Hashiguchi Y, Nakanishi K, Maekawa K, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012;36:193–201.
Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89:328–36.
Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–94.
Akaike H. Information theory and an extension of the maximum likelihood principle. Budapest: Akademia Kiado; 1973.
R Development Core Team. A language and environment for statistical computing. R. Foundation for Statistical Computing; 2006.
Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455–61.
Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, et al. Management of early invasive colorectal cancer-risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38:1286–95.
Suzuki T, Sadahiro S, Mukoyama S, Ishikawa K, Yasuda S, Tajima T, et al. Risk of lymph node and distant metastases in patients with early invasive colorectal cancer classified as Haggitt’s level 4 invasion. Dis Colon Rectum. 2003;46:203–8.
Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Uemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010;23:1068–72.
Araki Y, Isomoto H, Shirouzu K, Miura K, Iwanaga H, Okita A, et al. Clinicopathological characteristics of colorectal submucosal carcinoma with lymph node metastasis. Kurume Med J. 1993;40:123–7.
Hase K, Shatney CH, Mochizuki H, Johnson DL, Tamakuma S, Vierra M, et al. Long-term results of curative resection of “minimally invasive” colorectal cancer. Dis Colon Rectum. 1995;38:19–26.
Egashira Y, Yoshida T, Hirata I, Hamamoto N, Akutagawa H, Takeshita A, et al. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol. 2004;17:503–11.
Liang P, Hong J-W, Ubukata H, Liu G, Katano M, Motohashi G, et al. Myofibroblasts correlate with lymphatic microvessel density and lymph node metastasis in early-stage invasive colorectal carcinoma. Anticancer Res. 2005;25:2705–12.
Oh-e H, Tanaka S, Kitadai Y, Shimamoto F, Yoshihara M, Haruma K. Angiogenesis at the site of deepest penetration predicts lymph node metastasis of submucosal colorectal cancer. Dis Colon Rectum. 2001;44:1129–36.
Sakuragi M, Togashi K, Konishi F, Koinuma K, Kawamura Y, Okada M, et al. Predictive factors for lymph node metastasis in T1 stage colorectal carcinomas. Dis Colon Rectum. 2003;46:1626–32.
Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaboration study. J Gastroenterol. 2004;39:534–43.
Prall E. Tumour budding in colorectal carcinoma. Histopathology. 2007;50:151–62.
Lugli A, Karamitopoulou E, Zlobec I. Tumour budding: a promising parameter in colorectal cancer. Br J Cancer. 2012;106:1713–7.
Kaneko I, Shinji S, Oka S, Kawamura T, Hiyama T, Ito M, et al. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis Colon Rectum. 2006;50:13–21.
Kaneko I, Tanaka S, Oka S, Yoshida S, Hiyama T, Arihiro K, et al. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol. 2007;28:3829–35.
Yamauchi H, Togashi K, Kawamura Y, Horie H, Sasaki J, Tsujinaka S, et al. Pathological predictors for lymph node metastasis in T1 colorectal cancer. Surg Today. 2008;38:905–10.
Suzuki A, Togashi K, Nokubi M, Koinuma K, Miyakura Y, Horie H, et al. Evaluation of venous invasion by elastica van Gieson stain and tumor budding predicts local and distant metastases in patients with T1 stage colorectal cancer. Am J Surg Pathol. 2009;33:1601–7.
Komori K, Hirai T, Kanemitsu Y, Shimizu Y, Sano T, Ito S, et al. Is “depth of submucosal invasion >=1,000 mm” an important predictive factor for lymph node metastasis in early invasive colorectal cancer (PT1)? Hepatogastroenterology. 2010;57:1123–7.
Nakadoi K, Tanaka S, Kanao H, Terasaki M, Takata S, Oka S, et al. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol. 2012;27:1057–62.
Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura S-I, et al. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer (IARC); 2010. p. 134–46.
Jass JR, O’Brien MJ, Riddell RH, Snover DC. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma. Hum Pathol. 2007;38:537–45.
The Royal College of Pathologists of Australasia. Colorectal cancer structured reporting protocol, 1st edn. 2010. http://www.rcpa.edu.au. Accessed Feb 19 2013.
Williams GT, Quirke P, Shepherd NA, The Royal College of Pathologists. Standards and datasets for reporting cancers. Dataset for colorectal cancer, 2nd edn. 2007. http://www.rcpath.org/publications-media/publications/datasets/colorectal-cancer.htm. Accessed Feb 19 2013.
Kazama S, Watanabe T, Ajioka Y, Kanazawa T, Nagawa H. Tumour budding at the deepest invasive margin correlates with lymph node metastasis in submucosal colorectal cancer detected by anticytokeratin antibody CAM5.2. Br J Cancer. 2006;94:293–8.
Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Yokoo T, Ishii T. Histopathologic determinants of regional lymph node metastasis in early colorectal cancer. Cancer. 2008;112:924–33.
Ogawa T, Yoshida T, Tsuruta T, Tokuyama W, Adachi S, Kikuchi M, et al. Tumor budding is predictive of lymphatic involvement and lymph node metastases in submucosal invasive colorectal adenocarcinomas and in non-polypoid compared with polypoid growths. Scand J Gastroenterol. 2009;44:605–14.
Horcic M, Koelzer VH, Karamitopoulou E, Terracciano L, Puppa G, Zlobec I, et al. Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. Hum Pathol. 2012 (Epub ahead of print).
Puppa G, Senore C, Sheahan K, Vieth M, Lugli A, Zlobec I, et al. Diagnostic reproducibility of tumour budding in colorectal cancer: a multicentre, multinational study using virtual microscopy. Histopathology. 2012 (Epub ahead print).
Acknowledgments
The authors thank Professor Hidetaka Mochizuki who served as President of the 75th meeting of the Japanese Society for Cancer of the Colon and Rectum (JSCCR) for his valuable advice on this study. The authors also thank following investigators who participated in this study by offering their institutional data: Keisuke Minamimura (Mitsui Memorial Hospital), Munenori Ide (Gunma University), Yoshikazu Koide (Fujita Health University School of Medicine), Fumio Konishi (Saitama Medical Center, Jichi Medical University), Hiroshi Iino (Yamanashi Medical University), Soichi Tanaka (Matsuda Hospital Colo-proctological Institute), Mitsuo Kishimoto (Kyoto Prefectural University of Medicine), Tadahiko Masaki (Kyorin University School of Medicine), Keizo Yamaguchi (Kurume University Medical Center), Shinji Tanaka and Koichi Nakadoi (Hiroshima University Hospital), Hideto Fujita (Kanazawa University Hospital), Shiro Adachi and Taishi Hata (Toyonaka Municipal Hospital), Sachio Yokoyama (Kumamoto City Hospital), Shingo Kameoka and Takuzo Hashimoto (Tokyo Women’s Medical University), Yusuke Kinugasa (Shizuoka Cancer Center Hospital), Hiroyoshi Takemoto (Sakai Municipal Hospital), Takeyasu Suda (The Nippon Dental University Medical Hospital), Koji Nagata (Saitama Medical University).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueno, H., Hase, K., Hashiguchi, Y. et al. Novel risk factors for lymph node metastasis in early invasive colorectal cancer: a multi-institution pathology review. J Gastroenterol 49, 1314–1323 (2014). https://doi.org/10.1007/s00535-013-0881-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0881-3