Abstract
Purpose
The aim of this study was to evaluate the nutrition and metabolism status alteration during immunotherapy in advanced hepatocellular carcinoma (HCC) patients.
Methods
Patients with advanced HCC who participated in the clinical trials of single-agent anti-PD-1 immunotherapy or sorafenib were retrospectively included. We analyzed self-comparison of the nutritional and metabolic indices of patients in the anti-PD-1 and sorafenib treatment group. We conducted mutual-comparison of the mentioned indices between the disease progression group and disease control group among anti-PD-1 treatment patients. We further analyzed those indices with statistical differences by partial correlation and survival analysis.
Results
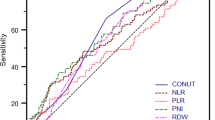
Both self-comparison before and after treatment in the anti-PD-1 group and mutual-comparison of disease progression and the control group showed significant differences in multiple indices, but we did not observe significant differences in the sorafenib group. Strikingly, albumin (ALB)/prognostic nutritional index (PNI, calculated by serum albumin and lymphocyte count) decreased distinctly in the immunotherapy disease progression group patients. However, changes in ALB/PNI were not significant in disease progression patients from the sorafenib group or in the disease control patients with immunotherapy. Partial correlation analysis suggested that ALB and PNI were positively correlated with the efficacy of immunotherapy. Furthermore, survival analysis showed that the median progression-free survival and median overall survival of patients in the ALB/PNI decreased group were significantly shorter than those of patients from the ALB/PNI increased group.
Conclusion
Anti-PD-1 immunotherapy might alter the nutritional and metabolic status in advanced HCC patients. We also should pay attention to the nutritional and metabolic status of patients when drug resistance is detected.



Similar content being viewed by others
Abbreviations
- PD-1:
-
Programmed death-1
- HCC:
-
Hepatocellular carcinoma
- BMI:
-
Body mass index
- AFP:
-
Alpha-fetoprotein
- WBC:
-
White blood cell
- N:
-
Neutrophil
- L:
-
Lymphocyte
- M:
-
Mononuclear
- E:
-
Eosinophils
- B:
-
Basophils
- RBC:
-
Red blood cell
- Hb:
-
Hemoglobin
- PLT:
-
Platelet
- TP:
-
Total protein
- ALB:
-
Albumin
- GLOB:
-
Globulin
- A/G:
-
Albumin/globulin ratio
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AKP:
-
Alkaline phosphatase
- TBA:
-
Total bile acid
- TB:
-
Total bilirubin
- DB:
-
Direct bilirubin
- IB:
-
Indirect bilirubin
- ADA:
-
Adenylate dehydrogenase
- GGT:
-
γ-glutamyl transcriptase
- Ccr:
-
Creatinine clearance rate
- Cr:
-
Creatinine
- BUN:
-
Urea nitrogen
- UA:
-
Uric acid
- LDH:
-
Lactate dehydrogenase
- AFU:
-
α-L-fucosidase
- GPDA:
-
Glycylproline dipeptidyl aminopeptidase
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- VLDL:
-
Very low-density lipoprotein
- GLU:
-
Glucose
- K:
-
Potassium
- Na:
-
Sodium
- Cl:
-
Chloride
- Ca:
-
Calcium
- P:
-
Phosphorus
- PNI:
-
Prognostic nutritional index
- PLR:
-
Platelet-to-lymphocyte ratio
- LMR:
-
Lymphocyte-to-monocyte ratio
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, SHARP Investigators Study Group (2008) Sorafenib in advanced hepatocellular car-cinoma. N Engl J Med 359:378–390
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173
Medavaram S, Zhang Y (2018) Emerging therapies in advanced hepatocellular carcinoma. Exp Hematol Oncol 7:17
El-Khoueiry AB, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502
Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, Bai Y, Yang L, Zhu H, Fang W, Lin X, Chen X, Li E, Wang L, Chen C, Zou J (2020) Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 21(4):571–580
Yen CJ, Markman B, Chao Y et al (2017) Preliminary results of a phase 1A/1B study of BGB-A317, an anti-PD-1 monoclonal antibody (mAb), in patients with advanced hepatocellular carcinoma (HCC). Ann Oncol 28(Suppl 3):iii54
Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C (2017) Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis. Nutr Cancer 69(8):1151–1176
Boroughs LK, DeBerardinis RJ (2015) Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 17(4):351–359
Fearon KC, Glass DJ, Guttridge DC (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16(2):153–166
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85(9):1001–1005
Mohri T, Mohri Y, Shigemori T, Takeuchi K, Itoh Y, Kato T (2016) Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World J Surg Oncol 14(1):170
Matsumoto Y, Zhou Q, Kamimura K, Moriyama M, Saijo Y (2018) The prognostic nutrition index predicts the development of hematological toxicities in and the prognosis of esophageal cancer patients treated with cisplatin plus 5-fluorouracil chemotherapy. Nutr Cancer 70(3):447–452
Shimizu T, Taniguchi K, Asakuma M et al (2019) Lymphocyte-to-monocyte ratio and prognostic nutritional index predict poor prognosis in patients on chemotherapy for unresectable pancreatic cancer. Anticancer Res 39(4):2169–2176
Feng JR, Qiu X, Wang F et al (2017) Diagnostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Crohn’s disease. Gastroenterol Res Pract 2017:3526460
Qi Y, Liao D, Fu X, Gao Q, Zhang Y (2019) Elevated platelet-to-lymphocyte corresponds with poor outcome in patients with advanced cancer receiving anti-PD-1 therapy. Int Immunopharmacol 74:105707
Goh BKP (2016) Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (10cm) hepatocellular carcinoma. J Surg Oncol 113:621–627. https://doi.org/10.1002/jso.24197
Ren M, Li J, Xue R, Wang Z, Coll SL, Meng Q (2019) Liver function and energy metabolism in hepatocellular carcinoma developed in patients with hepatitis B-related cirrhosis. Medicine (Baltimore) 98(19):e15528
Ye Q, Yin W, Zhang L, Xiao H, Qi Y, Liu S, Qian B, Wang F, Han T (2017) The value of grip test, lysophosphatidlycholines, glycerophosphocholine, ornithine, glucuronic acid decrement in assessment of nutritional and metabolic characteristics in hepatitis B cirrhosis. PLoS One 12(4):e0175165
Alwarawrah Y, Kiernan K, MacIver NJ (2018) Changes in nutritional status impact immune cell metabolism and function. Front Immunol 9:1055
Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ (2014) Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol 192(1):136–144
Saha S, Shalova IN, Biswas SK (2017) Metabolic regulation of macrophage phenotype and function. Immunol Rev 280(1):102–111
MacIver NJ, Michalek RD, Rathmell JC (2013) Metabolic regulation of T lymphocytes. Annu Rev Immunol 31:259–283
Michalek RD, Rathmell JC (2010) The metabolic life and times of a T-cell. Immunol Rev 236:190–202
Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y (2009) Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 460(7251):103–107
Bruix J, Sherman M, American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022
Selçuk Ö, Yayla V, Çabalar M, Güzel V, Uysal S, Gedikbaşi A (2014) The relationship of serum S100B levels with infarction size and clinical outcome in acute ischemic stroke patients. Noro Psikiyatr Ars 51(4):395–400
Xiang QF, Zhan MX, Li Y, Liang H, Hu C, Huang YM, Xiao J, He X, Xin YJ, Chen MS, Lu LG (2019) Activation of MET promotes resistance to sorafenib in hepatocellular carcinoma cells via the AKT/ERK1/2-EGR1 pathway. Artif Cells Nanomed Biotechnol 47(1):83–89
Gouirand V, Guillaumond F, Vasseur S (2018) Influence of the tumor microenvironment on cancer cells metabolic reprogramming. Front Oncol 8:117
Choi Y, Kim JW, Nam KH, Han SH, Kim JW, Ahn SH, Park DJ, Lee KW, Lee HS, Kim HH (2017) Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer 20(4):602–611
Chang CH, Qiu J, O'Sullivan D et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162(6):1229–1241
Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, Li Y, Li G, Xiong W, Guo C, Zeng Z (2019) Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 18(1):10
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, Liu X (2019) Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J 17:661–674
Xu F, Jin T, Zhu Y, Dai C (2018) Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res 37(1):110
Cai J, Qi Q, Qian X, Han J, Zhu X, Zhang Q, Xia R (2019) The role of PD-1/PD-L1 axis and macrophage in the progression and treatment of cancer. J Cancer Res Clin Oncol 145(6):1377–1385
Benjamin DI, Cravatt BF, Nomura DK (2012) Global profiling strategies for mapping dysregulated metabolic pathways in cancer. Cell Metab 16(5):565–577
Hirschey MD, DeBerardinis RJ, Diehl A, Drew JE, Frezza C, Green MF, Jones LW, Ko YH, le A, Lea MA, Locasale JW, Longo VD, Lyssiotis CA, McDonnell E, Mehrmohamadi M, Michelotti G, Muralidhar V, Murphy MP, Pedersen PL, Poore B, Raffaghello L, Rathmell JC, Sivanand S, Vander Heiden MG, Wellen KE, Target Validation Team (2015) Dysregulated metabolism contributes to oncogenesis. Semin Cancer Biol 35(Suppl):S129–S150
Antoun S, Khan S, Raynard B (2018) Managing malnutrition in cancer patients. Rev Prat 68(9):940–945
Tan CS, Read JA, Phan VH, Beale PJ, Peat JK, Clarke SJ (2015) The relationship between nutritional status, inflammatory markers and survival in patients with advanced cancer: a prospective cohort study. Support Care Cancer 23(2):385–391
Nicolini A, Ferrari P, Masoni MC, Fini M, Pagani S, Giampietro O, Carpi A (2013) Malnutrition, anorexia and cachexia in cancer patients: a mini-review on pathogenesis and treatment. Biomed Pharmacother 67(8):807–817
Menta PL, Correia MI, Vidigal PV, Silva LD, Teixeira R (2015) Nutrition status of patients with chronic hepatitis B or C. Nutr Clin Pract 30(2):290–296
Lebossé F, Gudd C, Tunc E, Singanayagam A, Nathwani R, Triantafyllou E, Pop O, Kumar N, Mukherjee S, Hou TZ, Quaglia A, Zoulim F, Wendon J, Dhar A, Thursz M, Antoniades CG, Khamri W (2019) CD8+T cells from patients with cirrhosis display a phenotype that may contribute to cirrhosis-associated immune dysfunction. EBioMedicine 49:258–268
Kim HY, Park JW (2017) Current immunotherapeutic strategies in hepatocellular carcinoma: recent advances and future directions. Ther Adv Gastroenterol 10(10):805–814
Zhang C, Wang H, Ning Z, Xu L, Zhuang L, Wang P, Meng Z (2016) Prognostic nutritional index serves as a predictive marker of survival and associates with systemic inflammatory response in metastatic intrahepatic cholangiocarcinoma. Onco Targets Ther 9:6417–6423
Sun J, Mei Y, Zhu Q, Shou C, Tjhoi WEH, Yang W, Yu H, Zhang Q, Liu X, Yu J (2019) Relationship of prognostic nutritional index with prognosis of gastrointestinal stromal tumors. J Cancer 10(12):2679–2686
Pinato DJ, North BV, Sharma R (2012) A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Br J Cancer 106(8):1439–1445
Königsbrügge O, Posch F, Riedl J, Reitter EM, Zielinski C, Pabinger I, Ay C (2016) Association between decreased serum albumin with risk of venous thromboembolism and mortality in cancer patients. Oncologist 21(2):252–257
Seebacher V, Grimm C, Reinthaller A, Heinze G, Tempfer C, Hefler L, Polterauer S (2013) The value of serum albumin as a novel independent marker for prognosis in patients with endometrial cancer. Eur J Obstet Gynecol Reprod Biol 171(1):101–106
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, Ferté C (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23(8):1920–1928
Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria JC, Ferté C (2018) Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 15(12):748–762
Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S (2019) Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 30(1):44–56
Funding
This study was supported by the National Natural Science Foundation of China Youth Program (Grant Nos. 81802350 and 81802355), Science and Technology Major Project of China (Grant No. 2017ZX10203205), and Zhejiang provincial medical and health young talents project of China (Grant No. 2019RC285).
Author information
Authors and Affiliations
Contributions
The study was designed by Weijia Fang, Yuefen Pan, Yizhen Jiang, Xiaoxuan Tu, Xiangying Zhang, Weiqin Jiang, Yi Zheng, Peng Zhao, Zhou Tong, and Qihan Fu. Material preparation, data collection, and analysis were performed by Yizhen Jiang, Xiaoxuan Tu, Xiangying Zhang, Haihong Liao, and Shuwen Han. Yuefen Pan and Weijia Fang led the study. The first draft of the manuscript was written by Yizhen Jiang, Shuwen Han, Quan Qi, Junjun Shen, Liping Zhong, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 76 kb)
Rights and permissions
About this article
Cite this article
Jiang, Y., Tu, X., Zhang, X. et al. Nutrition and metabolism status alteration in advanced hepatocellular carcinoma patients treated with anti-PD-1 immunotherapy. Support Care Cancer 28, 5569–5579 (2020). https://doi.org/10.1007/s00520-020-05478-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05478-x