Abstract
Purpose
Trastuzumab-based chemotherapy is usually administered through either a peripherally inserted central catheter (PICC) or a totally implanted vascular access device (PORT). As the most effective type of access is unknown, a feasibility trial, prior to conducting a large pragmatic trial, was undertaken.
Methods
The trial methodology utilized the integrated consent model incorporating oral consent. Patients receiving trastuzumab-based neo/adjuvant chemotherapy for early-stage breast cancer were randomized to a PICC or PORT insertion. Feasibility was reflected through a combination of endpoints; however, the a priori definition of feasibility was > 25% of patients approached agreed to randomization and > 25% of physicians approached patients. Secondary outcomes included rates of line-associated complications such as thrombotic events requiring anticoagulation, line infections or phlebitis.
Results
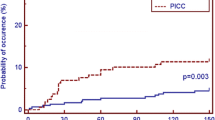
During the study period, 4/15 (26.7%) medical oncologists approached patients about study participation. Of 59 patients approached, 56 (94.9%) agreed to randomization, 29 (51.8%) were randomized to PICC and 27 (48.2%) to PORT access. Overall, 17.2% (5/29) and 14.8% (4/27) of patients had at least one line-associated complication in the PICC and PORT arms respectively. The study was terminated early due to slow accrual.
Conclusion
The study met its feasibility endpoints with respect to patient and physician engagement. However, the slow rate of accrual (56 patients in 2 years) means that conducting a large pragmatic trial would require additional strategies to make such a study possible.
Trial registration
ClinicalTrials.gov Identifier: NCT02632435

Similar content being viewed by others
References
Lipitz-Snyderman A et al (2015) Complications associated with use of long-term central venous catheters among commercially insured women with breast cancer. J Oncol Pract 11(6):505–510
Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, Flanders SA (2013) Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 382(9889):311–325
LeVasseur N, Stober C, Daigle K, Robinson A, McDiarmid S, Mazzarello S, Hutton B, Joy A, Fergusson D, Hilton J, McInnes M, Clemons M (2018) Optimizing vascular access for patients receiving intravenous systemic therapy for early-stage breast cancer-a survey of oncology nurses and physicians. Curr Oncol 25(4):e298–e304
LeVasseur N, Stober C, Ibrahim M, Gertler S, Hilton J, Robinson A, McDiarmid S, Fergusson D, Mazzarello S, Hutton B, Joy AA, McInnes M, Clemons M (2018) Perceptions of vascular access for intravenous systemic therapy and risk factors for lymphedema in early-stage breast cancer—a patient survey. Curr Oncol 25(4):e305–e310
Krein SL et al (2019) Patient-reported complications related to peripherally inserted central catheters: a multicentre prospective cohort study. BMJ Qual Saf 28(7):574–581
Robinson A et al (2018) Optimal vascular access strategies for patients receiving chemotherapy for early-stage breast cancer: a systematic review. Breast Cancer Res Treat:1–14
Robinson A et al (2019) A multicentre, randomized pilot trial comparing vascular access strategies for early stage breast cancer patients receiving non-trastuzumab containing chemotherapy. Breast Cancer Res Treat
Hilton J et al (2016) Novel methodology for comparing standard-of-care interventions in patients with cancer. J Oncol Pract 12(12):e1016–e1024
Basulaiman B et al (2019) Creating a pragmatic trials program for breast cancer patients: Rethinking Clinical Trials (REaCT). Breast Cancer Res Treat
ClinicalTrials.gov. Randomized trial standard of care vascular access strategies for (neo)adjuvant trastuzumab-based breast cancer treatment (OTT 15-06) NCT02632435. 2015 [cited 2018 August 1]; Available from: https://clinicaltrials.gov/ct2/show/NCT02632435
Kim SY, Miller FG (2014) Informed consent for pragmatic trials--the integrated consent model. N Engl J Med 370(8):769–772
Sugarman J, Califf RM (2014) Ethics and regulatory complexities for pragmatic clinical trials. JAMA 311(23):2381–2382
Singh KR, Agarwal G, Nanda G, Chand G, Mishra A, Agarwal A, Verma AK, Mishra SK, Goyal P (2014) Morbidity of chemotherapy administration and satisfaction in breast cancer patients: a comparative study of totally implantable venous access device (TIVAD) versus peripheral venous access usage. World J Surg 38(5):1084–1092
Di Carlo I et al (2001) Totally implantable venous access devices implanted surgically: a retrospective study on early and late complications. Arch Surg 136(9):1050–1053
Ozyuvaci E, Kutlu F (2006) Totally implantable venous access devices via subclavian vein: a retrospective study of 368 oncology patients. Adv Ther 23(4):574–581
Aw A, Carrier M, Koczerginski J, McDiarmid S, Tay J (2012) Incidence and predictive factors of symptomatic thrombosis related to peripherally inserted central catheters in chemotherapy patients. Thromb Res 130(3):323–326
Vescia S, Baumgärtner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, Harbeck N (2008) Management of venous port systems in oncology: a review of current evidence. Ann Oncol 19(1):9–15
Showalter SL, Brown JC, Cheville AL, Fisher CS, Sataloff D, Schmitz KH (2013) Lifestyle risk factors associated with arm swelling among women with breast cancer. Ann Surg Oncol 20(3):842–849
Piran S, Ngo V, McDiarmid S, le Gal G, Petrcich W, Carrier M (2014) Incidence and risk factors of symptomatic venous thromboembolism related to implanted ports in cancer patients. Thromb Res 133(1):30–33
Suleman A et al (2019) Implanted vascular access device related deep vein thrombosis in oncology patients: a prospective cohort study. Thromb Res 177:117–121
McDiarmid S, Scrivens N, Carrier M, Sabri E, Toye B, Huebsch L, Fergusson D (2017) Outcomes in a nurse-led peripherally inserted central catheter program: a retrospective cohort study. CMAJ Open 5(3):E535–e539
Tolaney SM et al (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372(2):134–141
Funding
Funding of this study was through the Rethinking Clinical Trials (REaCT program).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that this study complies with ethical standards in Canada. The study was approved by the provincial Research Ethics Board (Ontario Research Ethics Board, OCREB) and local REBs.
Conflict of interest
Dr. Awan reports participating in the Novartis Canada Advisory Board on the use of Ribociclib. Dr. Hutton reports personal fees from Cornerstone Research, outside the submitted work. The remaining authors declare that they have no conflicts of interest (Clemons, Stober, Kehoe, Bedard, MacDonald, Brunet, Saunders, Vandermeer, Mazzarello, Basulaiman, Robinson, Mallick, and Fergusson).
Research involving human participants
The study was approved by the appropriate institutional research ethics committees and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Clemons, M., Stober, C., Kehoe, A. et al. A randomized trial comparing vascular access strategies for patients receiving chemotherapy with trastuzumab for early-stage breast cancer. Support Care Cancer 28, 4891–4899 (2020). https://doi.org/10.1007/s00520-020-05326-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05326-y