Abstract
Purpose
The recent increase in emerging novel therapies in the bladder cancer therapeutic area has increased the need for fit-for-purpose patient-reported outcome (PRO) measures for these patients. This study evaluates the psychometric properties of the Functional Assessment of Cancer Therapy-Bladder (FACT-Bl) in 182 patients with advanced urothelial cancer (UC) and fills an important gap by demonstrating its validity for use in clinical trials.
Methods
Data were collected as part of a multicentre, open-label study of durvalumab in patients with inoperable or metastatic solid tumours. Psychometric properties evaluated include item and subscale characteristics (including correlation analysis), reliability (estimated using Cronbach’s α), validity (by independent sample t test), responsiveness (using mixed models with repeated measures), and clinically meaningful changes using both anchor-based and distribution-based methods.
Results
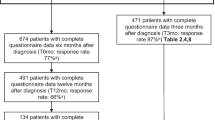
One hundred and seventy-two patients completed the FACT-Bl questionnaire at baseline. Many individual items had floor or ceiling effects indicative of minimal symptoms and high functioning. The psychometric properties of the existing established scales were assessed and found to range from acceptable to very good. Internal consistency (most Cronbach’s α coefficients range 0.66–0.85) and stability (test–retest reliability) generally exceeded standards for good reliability (most estimated intraclass correlations [ICCs] exceeded 0.70, although ICCs for some items [e.g. emotional well-being, ICC 0.58; social well-being, ICC 0.66] were lower than 0.70). Evidence for known-group validity of relevant FACT-Bl subscales and total score was demonstrated by significant differences between groups defined by baseline tumour burden and quality of life scores (difference of FACT-Bl total between low/high tumour burden groups 11.72 (p = 0.001); difference between low/high QoL scores groups 30.51 (p < 0.0001)). The FACT-Bl subscale and total scores were responsive to changes in bladder cancer symptom severity. Clinically meaningful changes in FACT-Bl scores were estimated.
Conclusions
Results support the use of the FACT-Bl within this patient population in future clinical research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2018, there were approximately 549,000 new cases of bladder cancer worldwide, and bladder cancer accounted for approximately 200,000 deaths [1]. The vast majority of bladder cancer cases (90%) were urothelial carcinoma [2, 3].
The burden of advanced urothelial cancer (UC) is attributable to disease and treatment characteristics [4, 5]. Haematuria, urinary frequency and urgency, and pain are among the most common signs and symptoms [6]. Additionally, symptoms such as bleeding, pain, dysuria, constipation, fatigue, emotional distress, and urinary obstruction adversely impact QoL in advanced bladder cancer [5]. Treatment-related side effects of fatigue, and the impact on daily activities, are also reported as relevant to these patients [5], as well as issues with self-esteem, embarrassment, and difficulty engaging in sexual relationships [4, 7, 8]. Emerging novel treatments [9] have accelerated interest in developing and validating patient-reported outcome (PRO) collection instruments to gain a full understanding of UC and disease impact, information important for patients and clinicians. In addition, PRO data can inform the benefit/risk assessment for regulators and payers [10].
Many instruments exist to assess health-related quality-of-life (HRQoL) in UC [11, 12]. PRO data are particularly important for these patients due to the burden of disease and therapy [11]. The Functional Assessment of Cancer Therapy-Bladder (FACT-Bl) has been used in several studies, often to determine comparative effects of various interventions on HRQoL [6,7,8, 13,14,15,16]. Despite its wide use, validity data in patients with locally advanced or metastatic UC are not published. The current study confirms the validity of the FACT-Bl in patients with urothelial carcinoma and follows an approach that is consistent with FDA guidelines for PRO validation [17]. It assesses the psychometric properties of the FACT-Bl in a group of patients who participated in the combined phase 1/2 clinical trial of durvalumab monotherapy. Funding for this research was provided by AstraZeneca.
Materials and methods
Study design
A multicentre, open-label (dose-escalation, dose-exploration, and dose-expansion) study was previously conducted to evaluate durvalumab’s safety, tolerability, and antitumour activity in patients with inoperable or metastatic solid tumours (Study 1108; NCT01693562). Study results are published elsewhere [18]. A total of 182 patients with upper and lower tract UC who received and have progressed or are refractory to 1 or 2 prior lines of systemic therapy for inoperable or metastatic disease, including a standard platinum-based regimen, were included in that open-label study. That clinical study was conducted according to the Declaration of Helsinki and approved by the independent ethics committee or institutional review board at each participating centre, with written informed consent obtained from all patients.
Data collection
PROs were evaluated using pen-and-paper versions of the FACT-Bl, the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire C30 (EORTC QLQ-C30), and a one-item pain questionnaire [19] completed at the screening visit: on day 1 of treatment doses 1, 3, and 5; at weeks 6, 12, and 16; and every 8 weeks until end of treatment (12 months).
Measures
FACT-Bl
The FACT-Bl (version 4) is a multidimensional, self-administered 39-item questionnaire to assess patient bladder cancer-specific symptoms using a ‘core’ set of questions (Functional Assessment of Cancer Therapy-General; FACT-G), a cancer site-specific bladder subscale, and HRQoL [20, 21]. Table 1 summarises the five subscales and three summary scores produced by the FACT-Bl.
NFBISI-18
The National Comprehensive Cancer Network FACT Bladder Symptom Index (NFBlSI-18), a measure of advanced bladder cancer-specific symptoms, assesses symptoms perceived as most important by patients and oncology clinical experts. The NFBISI-18 is based almost entirely on the FACT-Bl, including 16 items from the FACT-Bl instrument plus two items that have not been previously included (‘I feel weak all over’ and ‘I feel light-headed [dizzy]’) [5]. These two items were added based on qualitative analysis of patient and clinician priorities for symptoms and concerns associated with receiving treatment for advanced bladder cancer.
NFBlSI-18 yields three subscale scores (i.e. disease-related symptoms, treatment side effects, and general function/well-being) and a summary score. Items are rated on a 5-point Likert scale ranging from 0 = ‘not at all’ to 4 = ‘very much’ with a 7-day recall period. Higher scores represent better QoL. The subscale and summary scores are calculated using the Manual of Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System [22]. Only the NFBlSI-18 total summary score and the disease-related symptoms-physical subscale (NFBlSI-DRS-P) score are considered in the current analyses and prorated based on the 16 available items.
Statistical analysis
Psychometric analyses were performed on the full analysis set population using baseline (dose 1, day 1) data only, except for test–retest which used dose 3, day 29 and the responsiveness analysis where data up to dose 7, day 85 were used as period 2.
FACT-Bl item and scale characteristics
Performance of the 39 FACT-Bl items was evaluated by means of descriptive statistics (mean, standard deviation) and percentage of lowest and highest responses (floor and ceiling effects, respectively) at baseline (dose 1, day 1). Single items regarding pain and fatigue were prioritised in the clinical trial and examined specifically in psychometric analyses. Characteristics of FACT-Bl subscales for physical, functional, social/family, and emotional well-being and the Bladder Cancer subscale (PWB, FWB, SWB, EWB, and BlCS, respectively) as well as total summary scores (FACT-G total score, FACT-Bl total score, FACT-l Trial Outcome Index [TOI], and NFBlSI-18) were summarised using measures of central tendency (e.g. mean, median) and variability (e.g. standard deviation [SD], interquartile range).
Correlation analysis
Item-to-item, item-to-total, and between-scales correlations were assessed using Spearman correlation coefficients.
Reliability
Internal consistency reliability of subscale and total scores was estimated using Cronbach’s α. To evaluate reliability in stable patients, a group of patients whose EORTC-C30 QoL score was within ± 0.25 standard deviations of their baseline score was isolated. We evaluated intraclass correlation coefficients (ICCs) between baseline (period 1) and dose 3 (day 29) (period 2) [23]. Coefficients of 0.6 and higher are considered acceptable, and coefficients of 0.7 and higher are considered good [23].
Construct validity
Construct validity testing included convergent validity and known-group validity. Convergent validity was assessed at baseline using a Spearman correlation with EORTC QLQ-C30 domain scores. Known-group validity was examined by independent sample t test comparing baseline mean FACT-Bl scale scores by baseline tumour burden (above and below the median value, i.e. 59.9 mm) and by the baseline EORTC QLQ C-30 global health status/QoL score (above and below the median value, i.e. 50 points).
Responsiveness
The ability to detect change was assessed by comparing changes in the FACT-Bl scores over time between responders and non-responders using mixed models with repeated measures. Assessments up to and including dose 7 (day 85) were included in the analysis to maximise the longitudinal window and ensure sufficient sample size. Responders versus non-responders were defined in two ways: objective response (responders defined as patients with a confirmed objective complete or partial tumour response [18]; non-responders included the remainder of the patients [n = 150]) and patient evaluation of change using global health status (GHS)/QoL (patients demonstrating at least a 10-point improvement in GHS/QoL scale at dose 7 (day 85) compared with their baseline score were classified as responders and the remainder of patients as non-responders). The FACT-Bl was considered responsive if the mean change from baseline to dose 7 (day 85) is > 0 and statistically significant (indicating improvement) for the responder group and < 0 and statistically significant (indicating deterioration) for non-responders.
Clinically meaningful thresholds
As in other published studies [24], both anchor-based and distribution-based methods were used to explore a preliminary clinically meaningful change (CMC). For anchor-based methods, two external anchors using the objective response based on the Response Evaluation Criteria in Solid Tumours (RECIST) [25] criteria and the EORTC-C30 GHS/QoL scale at day 57 were used.
Several alternative methods were tested for convergence on the CMC using a robust sample size. Day 57 was selected as the most distant time point with at least 50% of the patients with a baseline reporting a score. The external anchors were as follows: (1) patients with objective response classified as ‘responders’, and the remaining patients as ‘non-responders’ and (2) patients classified as GHS/QoL responders/non-responders using the established clinical meaningful threshold of 10 points (as described above). Three distribution-based methods were also used: (1) a 0.5 SD, (2) 1 standard error of measurement (SEM) at visit baseline, and (3) reliable change index. T tests were performed to compare mean changes from baseline for clinical responders versus non-responders.
All data preparation and analyses were performed using SAS version 9.3 (SAS Institute, NC) or higher. Statistical comparisons were made using two-sided tests at α = 0.05 significance level unless specifically stated otherwise. Due to the exploratory nature of the analyses, adjustments for multiple comparisons were not made.
All analyses, except for item characteristics, were performed on items recoded as necessary with higher scores indicating better QoL.
Results
Baseline demographics and patient response
As of data cut-off (24 October 2016), 191 patients were treated for locally advanced or metastatic UC, 182 of which had progressed after platinum-based therapy [16]. Table 2 provides detailed patient demographics. Further demographic details are published elsewhere [18].
Questionnaire completion/compliance
Out of 182 patients in the second-line-or-later (2L+) post-platinum UC subgroup, 172 (94.5%) completed the FACT-Bl questionnaire at baseline. Response rate was high (over 92%) for all items. Two questions were for patients with ostomies only (46 [26%] patients answered these questions). Two questions about sexuality were asked: one for all patients (89 [49%] patients responded) and one for men only (65 [36%] patients responded). Compliance specific to eligible populations for these questions was not calculated.
Item and scale performance
Subject responses covered the entire range (0–4) for each FACT-Bl item. The majority of items had floor or ceiling effects reflecting minimal symptoms and high functioning. Issues were noted for the three items addressing sexual functioning where at least 25% of the patients reported the lowest response option.
Mean values for FACT-Bl and FACT-G total were 107.5 (range 45.7–156.0) and 75.6 (range 21.7–108.0), respectively, which represent 69% and 70%, respectively, of the scale range. The mean score for the FACT-Bl FWB scale was 15.8 (range 0.0–28.0), representing moderately impaired functioning. Subscales and summary scores at baseline are reported in Table 1.
Correlation analysis
The patterns of correlations matched expectations, with items in a scale correlating more highly with the score of that scale than with the score of other scales in the instrument, and all subscales correlating strongly with the FACT-Bl total score and FACT-G total score. The highest and second highest correlation coefficient was 0.91 observed between FWB and FACT-G and 0.81 observed between the PWB and FACT-G, respectively. All subscales correlated moderately or higher with the TOI or NFBlSI-18 index. The SWB subscale had very low correlations (r < 0.3) with the PWB subscale and moderate correlation with EWB and BlCS subscales. Table 3 shows the subscale and summary score correlations.
Reliability analysis
All subscales and summary scores demonstrate adequate to good internal consistency (Cronbach’s α range 0.63 to 0.93). The BlCS subscale internal consistency (Cronbach’s α value of 0.63) was slightly lower than the generally recommended 0.70 [26]. Composed of different symptoms, the BlCS subscale demonstrated more inter-item variability across patients.
Minimal change in the mean subscale or mean summary scores from baseline to dose 3 (day 29) demonstrated good test–retest reliability. The estimated ICC for the two visits (4 weeks apart) ranged from 0.58 (EWB) to 0.80 (FACT-G total score), with the lower bound of the 95% CI ranging from 0.45 (for EWB) to 0.85 (for FACT-G total score). All ICCs exceeded 0.70, except for emotional well-being (ICC 0.58) and social well-being (ICC 0.66). The mean ICC was 0.72. This confirms that FACT-Bl and the NFBlSI-18 showed fair to very good reliability for all dimensions in this patient population. The single items ‘I have a lack of energy’ and ‘I have pain’ also demonstrate acceptable reliability with ICC values of 0.60 and 0.70, respectively.
Construct validity
As could be expected, the physical functional domain from EORTC-QLQ C30 correlates highly (r ≥ 0.77) with the PWB. Strong correlations are observed also with the summary scores (FACT-BL total score, FACT-G total score, TOI and NFBLSI-18) and the FWB and BLCS. The EORTC-QLQ C30 emotional functional domain correlates highly (r ≥ 0.72) with EWB. In contrast, the EORTC-QLQ C30 social functional domain correlates weakly with SWB (r = 0.22).
The fatigue domain from EORTC-QLQ C30 correlates highly with the single-item GP1 (fatigue) from FACT-BL (r = − 0.8), and the pain domain from EORTC-QLQ C30 correlates highly with the single-item GP4 (pain) from FACT-BL (r = − 0.9). Note that the negative value of the Spearman correlation coefficient is due to the fact that higher scores on the fatigue and pain domains from EORTC-QLQ C30 indicate a worse health state while higher values on the single-items GP1 (fatigue) and GP4 (pain) indicate better health state.
The EORTC QLQ-C30 global health status/QoL score from EORTC-QLQ C30 correlates highly with FACT-G, FACT-TOI, FACT-BL total score, and NFBISI-18 (r > 0.76). Evidence for known-group validity was found for the FACT-Bl and NFBlSI-18 through significant differences between groups defined by baseline tumour burden and EORTC QLQ C-30 health status/QoL scores (Table 4). Increased tumour burden was associated with lower scores (worse health status and more symptoms). This finding holds for all the investigated scores except for the SWB and EWB where the difference between groups was not significant. Similarly, the FACT-Bl could show significant differences between groups defined by the GHS/QoL scores at baseline.
Responsiveness
The FACT-Bl subscale and total scores examined in this study were responsive to changes in bladder cancer symptom severity during a 12-week time frame. The mean change from baseline to dose 7 (day 85) was > 0 (indicating improvement) for almost all FACT-Bl scores for the responder group, while estimates < 0 (indicating deterioration) were observed for non-responders, regardless of which criterion was used to define responders (objective tumour response or EORTC QLQ C-30 GHS/QoL). The mean change from baseline to dose 7 (day 85) for responders using the GHS status/QoL ranged from 0.75 (for NFBlSI-18) to 21.1 (FACT-Bl total score), compared with − 2.95 (FACT-Bl total score) to 0.06 (BlCS) for non-responders. Similarly, the mean change from baseline to dose 7 (day 85) for responders using clinical measure objective response ranged from − 1.05 (for SWB) to 12.0 (FACT-Bl total score), compared with − 3.27 (FACT-Bl total score) to 0.16 (SWB) for non-responders.
Clinically meaningful change
The estimated clinically meaningful thresholds are provided in Table 5. Of note are the FACT-Bl total score ranges of 6.2–11.5 (rounded to 6–12), the FACT-Bl TOI of 5.4–8.7 (rounded to 5–9), the fatigue item (GP1) ranges of 0.6–1.1 (rounded to 1–2), the pain item (GP4) ranges of 0.7–1.0 (rounded to 1–2), and the NFBISI-18 ranges of 4.4–6.7 (rounded to 4–7).
The anchor-based estimates for clinically meaningful threshold estimates were larger and were reconciled with the distribution-based estimates to provide final estimates. Figure 1 shows the mean change for responders and non-responders in the clinical anchor group.
Discussion
This paper reports on the psychometric properties of the FACT-Bl in patients with locally advanced or metastatic UC. Our study fills a gap in the psychometric evidence for the FACT-Bl, following FDA guidelines for PRO validation [17], and may be useful for other studies of patients with advanced UC.
Results from the UC patient cohort Study 1108 showed clinically favourable activity and an acceptable safety profile for durvalumab [18]. The current analysis showed an overall high completion of the FACT-Bl and provided additional information on outcomes important to these patients. The completion rates were above the minimum required for scoring the scales and subscales.
The psychometric properties of the existing FACT-Bl scales and the pain and fatigue items were found to be very good, with correlations in the range of others accepted throughout the validation literature [27,28,29]. In addition to reliability, the FACT-Bl subscale and total scores showed good evidence for construct validity and were responsive to changes in UC symptom severity during a 12-week time frame assessed by both objective tumour response and patient evaluation of change, suggesting appropriateness of the instrument to detect symptomatic change.
This study has some limitations. First, the FACT-Bl was created several years ago, and many new therapies have been developed that address symptoms, and are associated with side effects, which are not necessarily captured in the FACT-Bl. Although this questionnaire does include an overall side-effect bother item (item GP5), it is possible that additional items may be needed to assess the impact of newer treatments on patients’ lives. Second, these data were obtained from patients participating in a clinical trial, and the results may not be completely generalisable to all patients with advanced UC.
The NFBlSI-18 is an abbreviated version of the FACT-Bl that adds two new questions to the 16 FACT-Bl questions that are deemed most important by patients with advanced cancer and by clinicians who care for them [5]. In this report, the total NFBlSI-18 score was prorated based on the 16 items in the FACT-Bl. So, although these results provide support for the use of the more-focused NFBlSI-18, more data on the 18-item version will be important to more fully understand its validity.
Another limitation of the study is the unavailability of anchors (e.g. the Patient Global Impression of Change [PGIC] [30]) to determine clinically meaningful thresholds. However, very useful clinical trial end point anchors were used, including tumour response data and the scores on the EORTC QLQ C-30, another commonly used QoL questionnaire. Our analysis suggests that the established thresholds of 2–3 points for the PWB, FWB, EWB, and SWB subscales and 5–7 points for FACT-G are appropriate for this patient population [31]. These ranges are comparable to those found in studies of patients with other cancers, including the prostate [32], lung [33], and breast [31].
The ICC coefficients showed good stability of the FACT-Bl. However, the use of the 29-day post assessment as a proximal measure for test–retest reliability may have attenuated the ICC coefficients, as real change may have occurred during this period. The ICCs for EWB and SWB did not exceed the threshold of 0.70, although ICCs for these two subscales are typically lower than for other subscales, as has been demonstrated in similar studies [34].
Conclusions
Psychometric properties of the existing established scales for the FACT-Bl as well as the NFBISI-18 were found to be very good for use in this population of advanced urothelial cancer patients. Emerging therapies for bladder cancer have accelerated interest in the development and validation of PRO instruments for this patient population to capture meaningful improvements in quality-of-life and symptom outcomes.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Leppert JT, Shvarts O, Kawaoka K, Lieberman R, Belldegrun AS, Pantuck AJ (2006) Prevention of bladder cancer: a review. Eur Urol 49(2):226–234. https://doi.org/10.1016/j.eururo.2005.12.011
Bladder cancer: introduction. https://www.cancer.net/cancer-types/bladder-cancer/introduction. Accessed 27 Feb 2019
Allareddy V, Kennedy J, West MM, Konety BR (2006) Quality of life in long-term survivors of bladder cancer. Cancer 106(11):2355–2362. https://doi.org/10.1002/cncr.21896
Jensen SE, Beaumont JL, Jacobsen PB, Abernethy A, Syrjala KL, Cella D (2013) Measuring priority symptoms in advanced bladder cancer: development and initial validation of a brief symptom index. J Support Oncol 11(2):86–93. https://doi.org/10.1016/j.suponc.2012.07.002
Jacobs BL, Lee CT, Montie JE (2010) Bladder cancer in 2010: how far have we come? CA Cancer J Clin 60(4):244–272. https://doi.org/10.3322/caac.20077
Gacci MSO, Cai T, Gore JL, D’Elia C, Minervini A, Masieri L, Giannessi C, Lanciotti M, Varca V, Simonato A, Serni S, Carmignani G, Carini M (2013) Quality of life in women undergoing urinary diversion for bladder cancer: results of a multicenter study among long-term disease-free survivors. Health Qual Life Outcomes 11:43
Zahran MH, Taha DE, Harraz AM, Zidan EM, El-Bilsha MA, Tharwat M, El Hefnawy AS, Ali-El-Dein B (2017) Health related quality of life after radical cystectomy in women: orthotopic neobladder versus ileal loop conduit and impact of incontinence. Minerva Urol Nefrol 69(3):262–270. https://doi.org/10.23736/S0393-2249.16.02742-9
Davarpanah NN, Yuno A, Trepel JB, Apolo AB (2017) Immunotherapy: a new treatment paradigm in bladder cancer. Curr Opin Oncol 29:184–195. https://doi.org/10.1097/cco.0000000000000366
Howie LJ SH, King-Kallimanis B, Roydhouse J, Theoret MR, Blumenthal GM, et al (2018) Patient-reported outcomes in PD-1/PD-L1 inhibitor registration trials: FDA analysis of data submitted and future directions. J Clin Oncol 36(Suppl 5S):Abstr 134
Danna BJ, Metcalfe MJ, Wood EL, Shah JB (2016) Assessing symptom burden in bladder cancer: an overview of bladder cancer specific health-related quality of life instruments. Bladder Cancer 2(3):329–340. https://doi.org/10.3233/BLC-160057
Tung-Ming LBE, Galsky M, Latini DM, Goltz HH, Lee CT, Quale DZ, Jensen BT, Girgis A, Given BA, Modamed NE (2017) Examining the psychometric properties of the bladder cancer needs assessment surgery. JOJ Urol Nephron 4(1). https://doi.org/10.19080/JOJUN.2017.4.555627
Gopalakrishna A, Longo TA, Fantony JJ, Harrison MR, Inman BA (2017) Physical activity patterns and associations with health-related quality of life in bladder cancer survivors. Urol Oncol 35(9):540 e541–540 e546. https://doi.org/10.1016/j.urolonc.2017.04.016
Herman JMSD, Montie J, Hayman JA, Sullivan MA, Kent E, Griffith KA, Esper P, Sandler HM (2004) Prospective quality-of-life assessment in patients receiving concurrent gemcitabine and radiotherapy as a bladder preservation strategy. Urology 64(1):69–73. https://doi.org/10.1016/j.urology.2004.02.024
Kikuchi E, Horiguchi Y, Nakashima J, Ohigashi T, Oya M, Nakagawa K, Miyajima A, Murai M (2006) Assessment of long-term quality of life using the FACT-BL questionnaire in patients with an ileal conduit, continent reservoir, or orthotopic neobladder. Jpn J Clin Oncol 36(11):712–716. https://doi.org/10.1093/jjco/hyl094
Li MY, Yang YL, Liu L, Wang L (2016) Effects of social support, hope and resilience on quality of life among Chinese bladder cancer patients: a cross-sectional study. Health Qual Life Outcomes 14:73. https://doi.org/10.1186/s12955-016-0481-z
Food and Drug Administration (2009) Guidance for industry, patient-reported outcome measures: use in medical product development to support labeling claims. U.S. Department of Health and Human Services, Silver Spring, MD
Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, Lee JL, Ong M, Sridhar SS, Vogelzang NJ, Fishman MN, Zhang J, Srinivas S, Parikh J, Antal J, Jin X, Gupta AK, Ben Y, Hahn NM (2017) Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol 3(9):e172411. https://doi.org/10.1001/jamaoncol.2017.2411
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J et al (1993) The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. J Clin Oncol 11(3):570–579. https://doi.org/10.1200/jco.1993.11.3.570
FACT-Bl (Version 4). www.facit.org/LiteratureRetrieve.aspx?ID=42242. Accessed 27 Feb 2019
Webster K, Cella D, Yost K (2003) The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 1(79):79. https://doi.org/10.1186/1477-7525-1-79
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2):420–428
Bharmal M, Fofana F, Barbosa CD, Williams P, Mahnke L, Marrel A, Schlichting M (2017) Psychometric properties of the FACT-M questionnaire in patients with Merkel cell carcinoma. Health Qual Life Outcomes 15(1):247. https://doi.org/10.1186/s12955-017-0815-5
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Taber K (2017) The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ 48:1273–1296. https://doi.org/10.1007/s11165-016-9602-2
Cella D, Kallich J, McDermott A, Xu X (2004) The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: results from five randomized clinical trials. Ann Oncol 15(6):979–986
Mallinson T, Cella D, Cashy J, Holzner B (2006) Giving meaning to measure: linking self-reported fatigue and function to performance of everyday activities. J Pain Symptom Manag 31(3):229–241. https://doi.org/10.1016/j.jpainsymman.2005.07.012
Victorson D, Soni M, Cella D (2006) Metaanalysis of the correlation between radiographic tumor response and patient-reported outcomes. Cancer 106(3):494–504. https://doi.org/10.1002/cncr.21637
Guy W (ed) (1976) ECDEU Assessment manual for psychopharmacology. US Department of Health, Education, and Welfare Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, Rockville
Yost KJ, Eton DT (2005) Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof 28(2):172–191. https://doi.org/10.1177/0163278705275340
Cella D, Nichol MB, Eton D, Nelson JB, Mulani P (2009) Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy—Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 12(1):124–129. https://doi.org/10.1111/j.1524-4733.2008.00409.x
Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, Wolf MK, Johnson DH (2002) What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol 55(3):285–295
Cicchetti D (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6(4):284–290
Acknowledgements
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. All human subjects provided written informed consent with guarantees of confidentiality. The authors would like to thank the patients, their families and caregivers, and all investigators involved in this study. Medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines, was provided by Molly Aldridge, MPH, Diane Kwiatkoski, PhD, and Eric Weathers of IQVIA (Research Triangle Park, NC, USA) and was funded by AstraZeneca.
Funding
This study was funded by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
That clinical study was conducted according to the Declaration of Helsinki and approved by the independent ethics committee or institutional review board at each participating centre, with written informed consent obtained from all patients.
Conflict of interest
David Cella, PhD reports non-financial support from FACIT.org and personal fees from GSK, outside the submitted work, and is a PI of a research grant from AstraZeneca unrelated to this study.
David Cella, PhD, and all authors have full access to the study data reported in this manuscript. On request of the journal editors, the data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Degboe, A., Ivanescu, C., Rohay, J.M. et al. Validity and performance of the Functional Assessment of Cancer Therapy-Bladder (FACT-Bl) among advanced urothelial cancer patients. Support Care Cancer 27, 4189–4198 (2019). https://doi.org/10.1007/s00520-019-04709-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04709-0